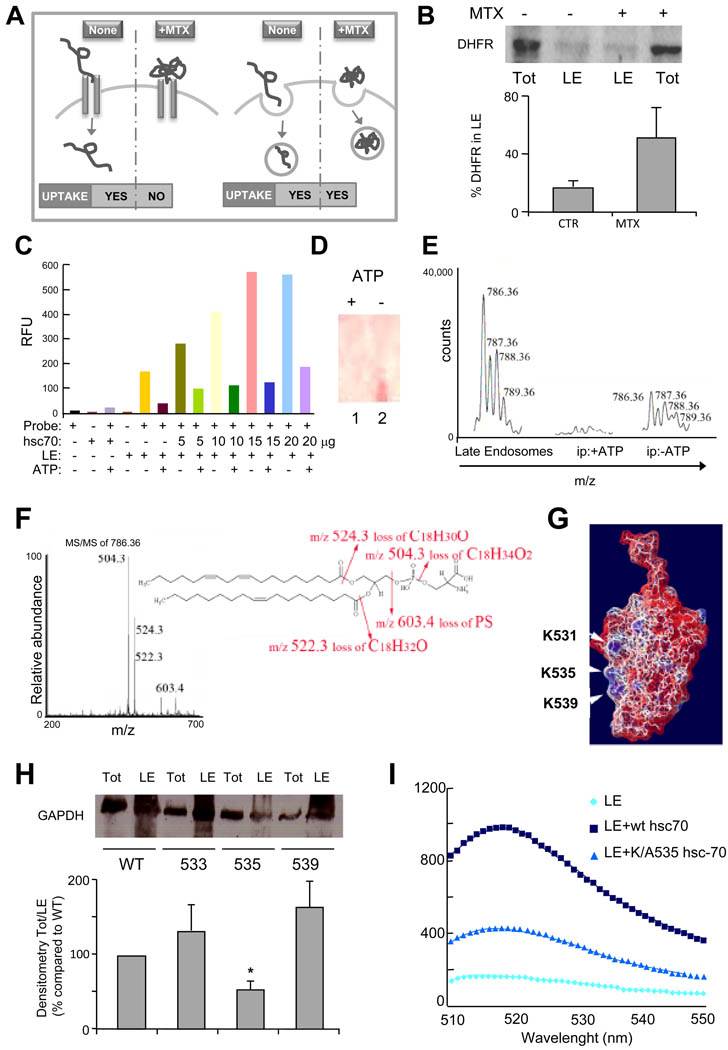
Figure 4. Recruitment of hsc70 to late endosomes is required for endosomal uptake of cytosolic proteins.
(A) Western blot analysis of total (Tot) and endosomal (LE) DHFR upon cellular treatment without or with methotrexate (MTX). (B) Schematic of hsc70-mediated DHFR translocation in lysosomes (left) and late endosomes (right) in presence and absence of MTX. (C) Fluorescence emission scans (excitation wavelength 497 nm, emission scans 510–550 nm) of hsc70 binding to fluorescence labeled LE in presence or absence of ATP. Graphic representation of the fluorescence maximum emission (Λ 520 nm) extrapolated from data reported in Figure S4. Experimental conditions in c and S4 are shown using the same color code. (D) Thin layer chromatography (staining with 0.1% ninhydrin) of lipids eluted from hsc70/LE immunoprecipitation performed in presence or absence of ATP. (E) MS analysis of lipids eluted from hsc70/LE immunoprecipitation performed in presence or absence of ATP. (F) MS/MS fragmentation of (E) and sequenced phosphatidylserine fragments. A molecular species with an m/z of 786.36 (first peak of the molecular envelope) was detected both in total LE (positive control) and in the hsc70 immunoprecipitate but only in the absence of ATP. (G) Electrostatic surface of the C terminal of hsc70 using the color code built in the software Swiss PDB Viewer (red-negative charges (acidic), blue-positive charges (basic) and white-neutral (hydrophobic)). The NMR solved three-dimensional structure of the substrate-binding domain of the mammalian chaperone protein hsc70 was used for all modeling studies (PDB ID: Hsc70)(Morshauser et al., 1999). (H) Western blot analysis of hsc70-myc present in late endosomal compartments following transfection of wild type or mutated hsc70. Ratio between total and endosomal hsc70-myc is expressed. (I) Fluorescence analysis of wild type and mutant hsc70 binding to LE. (See also Fig. S4).