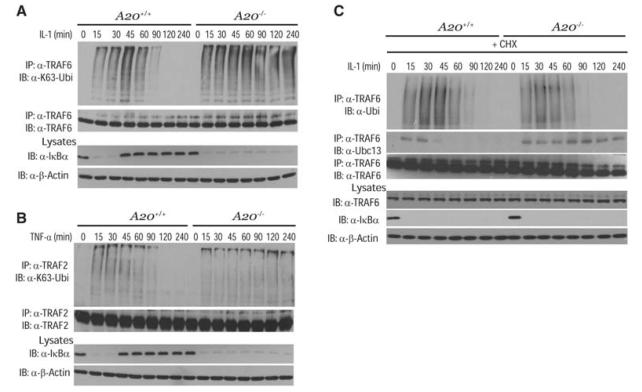
Fig. 2.
Negative regulation of TRAF2 and TRAF6 polyubiquitination by A20. (A) Kinetics of TRAF6 ubiquitination. A20+/+ and A20−/− MEFs were stimulated with IL-1 for the indicated times. Proteins from lysates were immunoprecipitated with antibody to TRAF6, eluted with 1% SDS, diluted in lysis buffer, reimmunoprecipitated with TRAF6 antibody followed by immunoblotting with K63-specific antibody to Ubi or antibody to TRAF6. Lysates were subjected to immunoblotting with antibodies to IκBα or β-actin. (B) Kinetics of TRAF2 ubiquitination. A20+/+ and A20−/− MEFs were stimulated with TNF-α for the indicated times. Proteins from lysates were immunoprecipitated with antibody to TRAF2, eluted with 1% SDS, diluted in lysis buffer, and reimmunoprecipitated with TRAF2 antibody, followed by immunoblotting with antibodies to Ubi or TRAF2. Lysates were subjected to immunoblotting with antibodies to IκBα and β-actin. (C) Stability of ubiquitinated TRAF6. A20+/+ and A20−/− MEFs were pretreated with cycloheximide, followed by IL-1 stimulation for the indicated times. Proteins from lysates were immunoprecipitated with antibody to TRAF6 as described (A), followed by immunoblotting with antibodies to Ubi, Ubc13, or TRAF6. Lysates were subjected to immunoblotting with antibodies to TRAF6, IκBα, and β-actin. The results shown are representative of three independent experiments.