Abstract
Retinal mitochondria become dysfunctional in diabetes and the production of superoxide radicals is increased; over-expression of MnSOD abrogates mitochondrial dysfunction and prevents the development of diabetic retinopathy. The mitochondrial DNA (mtDNA) is particularly prone to oxidative damage. The aim of this study is to examine the role of MnSOD in the maintenance of mtDNA. The effect of MnSOD mimic, MnTBAP or over-expression of MnSOD on glucose-induced alterations in mtDNA homeostasis and its functional consequence was determined in retinal endothelial cells. Exposure of retinal endothelial cells to high glucose increased mtDNA damage and compromised the DNA repair machinery. The gene expressions of mitochondrial-encoded proteins of the electron transport chain complexes were decreased. Inhibition of superoxide radicals by either MnTBAP or by over-expression of MnSOD prevented mtDNA damage and protected mitochondrial-encoded genes. Thus, the protection of mtDNA from glucose-induced oxidative damage is one of the plausible mechanisms by which MnSOD ameliorates the development of diabetic retinopathy.
Keywords: Diabetic retinopathy, DNA damage, endothelial cells, mitochondrial DNA, MnSOD
Introduction
Diabetic retinopathy is the leading cause of blindness in young adults. In the pathogenesis of diabetic retinopathy, retinal microvasculature undergoes many biochemical changes and the cells apoptosis is accelerated before early histopathological changes characteristic of diabetic retinopathy, including acellular capillaries and pericyte ghosts, are detected [1–3]. Previous work has suggested that hyperglycaemia-induced oxidative stress is one of the major contributors to the accelerated loss of retinal capillary cells in diabetes [4–10], but the exact mechanism remains elusive.
Retinal mitochondria become dysfunctional in diabetes and the production of superoxide radicals is increased [4,7]. This is further complicated by compromised antioxidant defense mechanisms including inactivation of mitochondrial-specific superoxide dismutase (MnSOD) and decreased levels of reduced glutathione (GSH). The release of cytochrome c from mitochondria is induced resulting in the apoptosis of retinal capillary cells [4,7,11,12]. Similar lesions are also observed in retinal endothelial cells exposed to a high glucose environment [4,6]. Inhibition of oxidative stress through over-expression of MnSOD abrogates many of these changes and prevents the development of retinopathy in diabetic mice [4,7,12], further substantiating the role of hyperglycaemia-induced mitochondrial dysfunction in the pathogenesis of diabetic retinopathy. However, the molecular mechanisms contributing to mitochondrial dysfunction in diabetes is yet to be fully elucidated.
Mitochondrial DNA (mtDNA) maintenance is vital to normal function as it encodes essential components of complexes of the electron transport chain and ribosomal and transfer RNAs necessary for protein production within the mitochondria [13]. In contrast to nuclear DNA (nDNA), which is tightly wrapped around histones, mtDNA is a circular double-stranded molecule packaged into nucleoid-like structure and is not well protected from insults. This, in addition to the close proximity of mtDNA to superoxide radicals generated by the electron transport chain, makes it particularly prone to oxidative damage [13,14]. Oxidative damage to mtDNA initiates a detrimental feed-back loop where the decreased production of mitochondrial-encoded sub-units, important in electron transport complexes, damage the electron transport system further increasing production of reactive oxygen species (ROS) [14–16]. To combat this and to maintain genome stability, mitochondria utilizes base excision repair (BER) as the primary DNA repair mechanism [17,18]. DNA repair enzyme 8-oxoguanine DNA glycosylase (OGG1) initiates the cycle and removes oxidative stress-induced damaged or inappropriate bases [19]. Mitochondrial dysfunction induced by high glucose in endothelial cells is considered to be related with mtDNA damage [9]. However, the role of mtDNA damage in retinal capillary cells, the site of histopathology, in the development of diabetic retinopathy is not clear.
The objective of this study is to examine the effect of hyperglycaemia on mtDNA maintenance in retinal endothelial cells and the role of MnSOD in protection of mtDNA damage.
Methods
Retinal endothelial cells
Endothelial cells (BRECs) were isolated from bovine retina and cultured on polystyrene dishes coated with 0.1% gelatin [4,20]. The cells from the 3rd to 6th passage were incubated in Dulbecco’s Modified Eagle Medium (DMEM) containing 2% heat inactivated foetal calf serum, 10% Nu-serum, 50 μg/ml heparin, 1 μg/ml endothelial growth supplement and antibiotic/anti-mycotic supplemented with 5 mM glucose or 20 mM glucose for 4–5 days in the presence or absence of a cell permeable MnSOD mimic MnTBAP (200 μM; from Biomol, Plymouth Meeting, PA) [4]. The medium and MnTBAP were replaced every other day. Osmotic controls included the cells incubated in identical experimental conditions with 20 mM mannitol instead of 20 mM glucose. At the end of the experimental period, cells were harvested. Mitochondria were prepared by differential centrifugation [4]. These cells exhibited their typical ‘cobblestone’ morphology.
Transient transfection of endothelial cells with MnSOD
Endothelial cells from the 3rd to 5th passage were transfected with 3 μg/ml MnSOD expression plasmid as previously described [6]. Briefly, transfection complexes were formed using Effectene transfection reagent (Qiagen, Valencia, CA) according to the manufacturer’s instructions and incubated for 8 h The cells were rinsed with DMEM and incubated in 5 mM (normal) or 20 mM (high) glucose media. Parallel incubations were carried out using the transfection reagent alone (Mock). The transfection was repeated at least four times using different endothelial cell preparations.
Assessment of DNA damage
Extended length polymerase chain reaction
DNA was isolated from BRECs with the DNeasy blood and tissue kit from Qiagen according to the manufacturer’s protocol and quantified using the Quant-iT dsDNA assay (Invitrogen, Carlsbad, CA). Mitochondrial or nuclear genome specific quantitative extended length PCR was performed with the GeneAmp XL PCR kit (Applied Biosystems, Foster City, CA) following the method described by others [21,22]. Briefly, 15 ng DNA was amplified in a reaction mixture containing 1× XL PCR buffer II, 200 μM dNTP, 1.1 mM Mg(OAc)2, and 0.1 μM genome specific primers (Table I) and 1 unit rTth DNA polymerase. Reaction was incubated for 1 min at 75°C prior to addition of polymerase, followed by 1 min at 94°C, 20 cycles of 94°C for 15 s and 65°C for 12 min and a final extension at 72°C for 12 min. PCR products were resolved by agarose gel electrophoresis and imaged with a UV transilluminator. Relative amplification was quantified by normalizing the intensity of the long product to the short product (mtDNA=13.4 kb/232 bp and nDNA=13.8 kb/241 bp).
Table I.
Bovine specific primers for detection of DNA damage and mitochondrial-encoded genes.
| Target | Primer sequence (5′ to 3′) | Product (base pairs) |
|---|---|---|
| mtDNA-long | 13 353 | |
| forward | ATG AGT TGG TAG TTT CGG TTG GGG TG | |
| reverse | ATT CTG TGG TCT GTG TAT GGG CGT GT | |
| mtDNA-short | 232 | |
| forward | CAT ACT CCT CTG TAA GCC ACA TAG C | |
| reverse | AGA CTT GCT AGT AGT CAT CAG GTG G | |
| nDNA-long | 13 823 | |
| forward | ACA TGC TCC AAC GGC TGC AA | |
| reverse | TGG CAG GGT CCG AAA ATT GCT | |
| nDNA-short | 241 | |
| forward | TAA GGC TCC CTG CCT CTA CA | |
| reverse | ACC CTT CAA CTT CCA CGA TG | |
| ND1 | 297 | |
| forward | AGG ACC ATT TGC CCT CTT CT | |
| reverse | GGT GGG ATG CCT GAT GTA AG | |
| ND4 | 269 | |
| forward | CCT ACA AAC GCT CCT TCC AC | |
| reverse | TGA GTG CAT TTT CCC GTG TA | |
| ND6 | 250 | |
| forward | CGT GAT AGG TTT TGT GGG GT | |
| reverse | GCC AGT AAC AAA TGC CCC TA | |
| Cytochrome b | 298 | |
| forward | CGA TAC ATA CAC GCA AAC GG | |
| reverse | AGA ATC GGG TAA GGG TTG CT | |
| β-actin | 205 | |
| forward | CCTCTATGCCAACACAGTGC | |
| reverse | CATCGTACTCCTGCTTGCTG |
Quantification of gene expression
Total RNA was isolated from BRECs using TRIzol reagent (Invitrogen), DNase-treated with RQ1 RNase-free DNAse (Promega, Madison, WI) and converted to cDNA using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). For real time RT-PCR, gene specific TaqMan assays (Applied Biosystems) and 90 ng cDNA were used to assess mRNA abundance of OGG1 by real-time RT-PCR; 18s rRNA served as an internal control. Fold-change in mRNA abundance was calculated with the ddCt method as routinely used by us [23].
For semi-quantitative RT-PCR, mitochondrial encoded genes were amplified from 0.5 μl cDNA with GoTaq DNA polymerase (Promega) and gene specific forward and reverse primers (Table I). After denaturation at 95°C for 2 min, amplification occurred using cycles of 95°C for 1 min, 50°C for 2 min and 72°C for 1 min followed by a final extension for 5 min at 72°C. Mitochondrial encoded genes (ND1, ND4, ND6 and cytochrome b) and β-actin were amplified with 28 cycles. PCR products were visualized by agarose gel electrophoresis and imaged with a UV transilluminator. Relative amplification was quantified by normalizing the gene specific band intensity to that of β-actin.
Immunofluorescence microscopy
Immunofluorescence microscopy was used to quantify the accumulation of superoxide radicals. Changes in mitochondrial inner membrane potential were determined using MitoTracker Red CM-H2XRos (Invitrogen) by taking the advantage of the sequestration of this fluorescent probe within the mitochondrial inner membrane as a function of electrical potential. After experimental treatment, the cells were rinsed with DMEM and incubated for 30 min at 37°C in 5 mM glucose medium containing 400 nM MitoTracker Red [24]. The cells were washed with PBS and fixed in 4% formaldehyde for 15 min and permeablized with 0.2% Triton-X 100 for 10 min. After blocking for 1 h with 1% BSA, the cells were incubated with OGG1 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at room temperature. They were then washed three times with 20 mM Tris-HCl buffer (pH 7.5) containing 0.9% NaCl and 0.05% tween 20, blocked again with 10% goat serum for 30 min and incubated with FITC-conjugated anti-rabbit IgG1 for 1 h. Following additional washing with PBS, cover-slips were mounted with Vectashield mounting media (Vector Laboratories; Burlingame, CA) and imaged using an Olympus BX50 fluorescence microscope fitted with a MagnaFIRE camera and MagnaFIRE software version 2.1C (Olympus America Inc., Melville, NY).
Mitochondrial oxidative stress
To further confirm mitochondrial oxidative stress and dysfunction, the levels of GSH were quantified using 5 μg mitochondrial protein by an enzymatic recycling method routinely used in our laboratory [7]. Mitochondrial protein was deproteinized by phosphoric acid and GSH concentration was measured in the deproteinized samples using DTNB (5, 5′-dithiobis-2-nitrobenzoic acid).
The collapse of mitochondrial membrane potential was quantified by measuring the swelling of the isolated mitochondria using a spectrophotometric method [7]. The decrease in absorbance at 540 nm induced by calcium chloride was followed for 3–5 min. The extent of swelling was calculated as a percentage of swelling with respect to the maximum swelling achieved by exposure to calcium chloride.
Statistical analysis
Data are expressed as mean ± standard deviation. Statistical analysis was performed using the non-parametric Kruskal-Wallis test followed by Mann-Whitney U-test. P < 0.05 was considered statistically significant.
Results
High glucose induces mtDNA damage in retinal endothelial cells
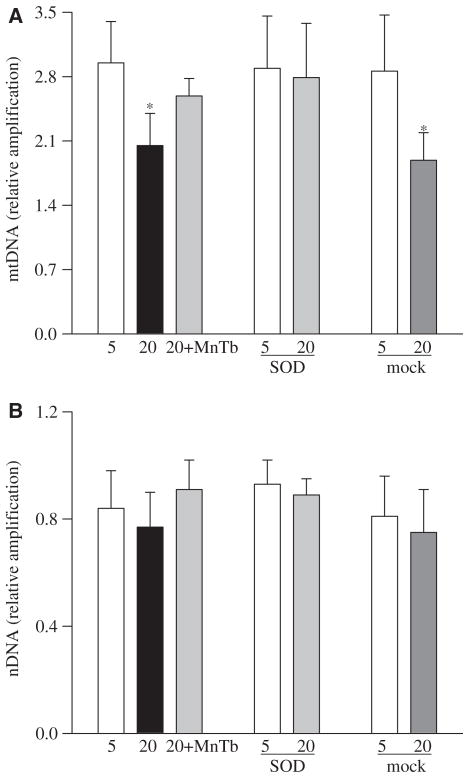
High glucose exposure of retinal endothelial cells resulted in an increase in mtDNA damage as detected by over 30% reduction in the amplification of mtDNA, as shown by extended length PCR (Figure 1A). In contrast, in the same cell preparations, high glucose had no significant effect on nuclear DNA (nDNA) damage (Figure 1B).
Figure 1.
Effect of high glucose on mitochondrial DNA damage: DNA damage was assessed using 15 ng total DNA and mitochondrial or nuclear specific primers for long and short PCR products in the cells incubated with 5 mM glucose or 20 mM glucose in the presence of MnTBAP or from the cells transfected with MnSOD or Mock cells incubated in 5 mM or 20 mM glucose media. (A) Relative amplification was calculated by normalizing the intensity of the 13.3 kb product to the 232 bp product for mtDNA and (B) the 13.8 kb product to the 241 bp product for nDNA. Each measurement was made in duplicate in at least three different cell preparations. 5=5 mM glucose, 20=20 mM glucose, 20+MnTb=20 mM glucose + 200 μM MnTBAP, SOD=cells transfected with MnSOD, Mock=cells treated with the transfection reagent alone. *p < 0.05 compared to the values obtained from the un-transfected cells incubated in 5 mM glucose.
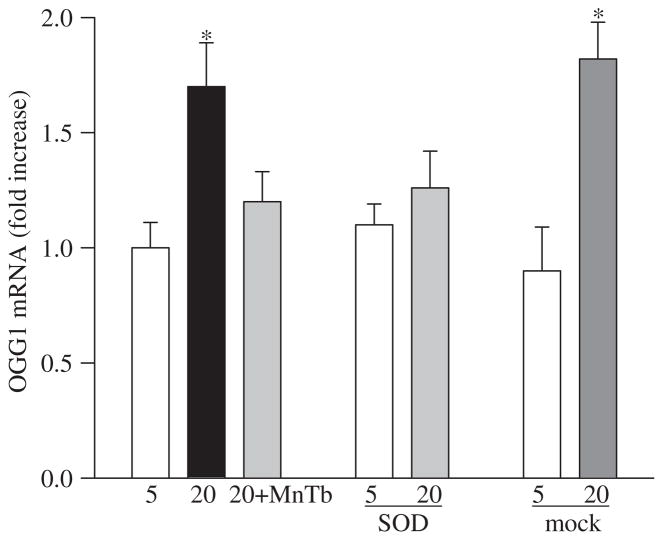
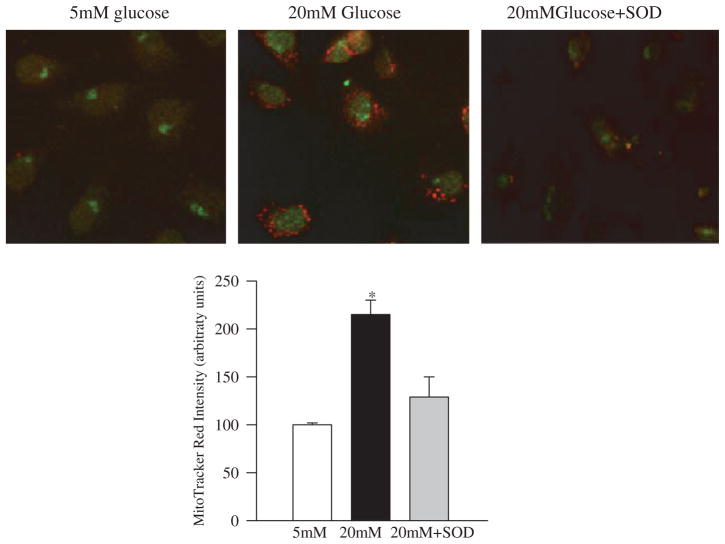
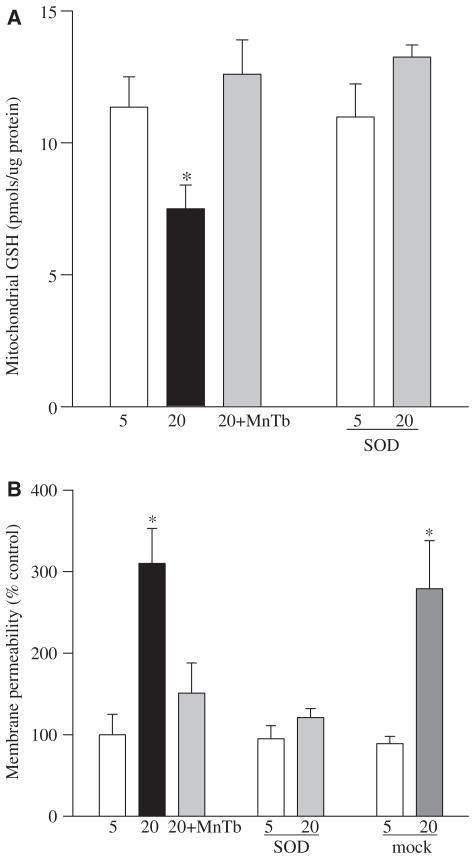
Since the DNA glycosylases OGG1 performs the critical step of base excision repair of recognition and removal of oxidatively modified bases [17], its role in mtDNA damage was pursued. Gene abundance of OGG1 was increased by 1.5–2.0-fold in the cells incubated in high glucose compared to the cells incubated in normal glucose (Figure 2), suggesting that the cell produces more OGG1 to overcome the mtDNA damage induced by high glucose. However, as shown by immunofluorescence microscopy (Figure 3), the nuclear abundance of OGG1 was significantly higher in cells incubated in high glucose compared to the cells incubated in normal glucose. Additional staining with MitoTracker Red CM-H2XRos, which is a mitochondrial-selective probe that only produces fluorescence upon oxidation, and its accumulation is directly related to membrane potential [25] not only confirmed the presence of increased oxidative stress in the cells exposed to high glucose, but also documented increased OGG1 staining in the nuclear components of the cells surrounded by MitoTracker Red staining. Increased mitochondrial oxidative stress was further confirmed by over 30% reduction in the levels of intracellular antioxidant, GSH, in the mitochondria (Figure 4A), and over 3-fold increase in the membrane permeability (Figure 4B).
Figure 2.
Effect of glucose on OGG1 and protection by MnSOD:RNA isolated from the cells treated with MnTBAP or transfected with MnSOD or treated with the transfection reagent alone and was assessed by real-time RT-PCR for OGG1. The values were normalized to 18 s mRNA in each sample. Fold-change relative to the values obtained from un-transfected cells incubated in 5 mM glucose was calculated using the ddCt method. Results are from the measurements made in three-to-five preparations. *p < 0.05 compared to control.
Figure 3.
Effect of MnSOD on the co-localization of superoxide radicals and OGG1 protein: at the end of incubation in high glucose (4 days) the cells were incubated with Mitotracker Red, washed and fixed with formaldehyde. The cells were permeabilized with Triton X and immunostained for OGG1 using FITC-conjugated secondary antibody. The picture is representative of five different experiments. The accompanying histogram represents the intensity of Mitotracker Red quantified by MetaMorph software in a constant area and constant threshold. 5 mM glucose and 20 mM glucose represent un-transfected cells incubated in 5 mM or 20 mM glucose and 20 mM glucose+SOD=MnSOD transfected cells incubated with 20 mM glucose for 4 days.
Figure 4.
Effect of MnSOD on mitochondrial GSH and membrane permeability: mitochondria were prepared from the cells transfected with MnSOD or untransfected, incubated in the presence or absence of MnTBAP. (A) GSH levels were quantified by a colourimetric method and (B) membrane permeability by a spectrophotometric method by measuring the decrease in absorbance at 540 nm induced by calcium chloride.
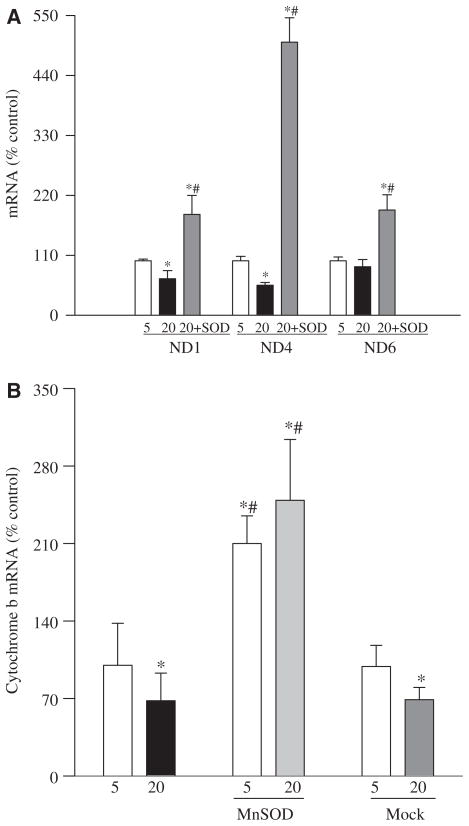
In order to determine the functional consequence of mtDNA damage, we investigated the effect of high glucose on the expressions of some of the electron transport chain proteins that are encoded by mtDNA. As shown in Figure 5 the gene expressions of ND1 and ND4 of complex I and cytochrome b of complex III were reduced by 30–50% in the cells incubated in high glucose compared to normal glucose.
Figure 5.
Effect of glucose on altered abundance of mitochondrial-encoded genes and their protection by MnSOD: transcript abundance was assessed in DNase-treated RNA isolated from retinal endothelial cells using conventional RT-PCR for ND1, ND4 and ND6 of complex I (A) and Cyt b of complex III (B) using the primers provided in Table I. Relative mRNA abundance was quantified using Un-Scan-It Gel digitizing software and the values in the figures are presented as mean band intensity of the target gene normalized by the intensity of β-actin. The values obtained from the un-transfected cells incubated in 5 mM glucose are considered 100% (control). Each measurement was made in four or more different cell preparations. *p < 0.05 compared to control, #p < 0.05 compared to 20 mM glucose alone.
Effect of quenching of superoxide radicals on glucose-induced mtDNA damage
To investigate the effect of amelioration of mitochondrial oxidative stress on glucose-induced mtDNA damage, we used both pharmacologic and genetic means to inhibit superoxide radicals.
As shown in Figure 1, inclusion of a MnSOD mimetic or over-expression of MnSOD decreased glucose-induced mtDNA damage; mtDNA long component amplification obtained from the cells incubated in 20 mM glucose supplemented with MnTBAP or cells over-expressing MnSOD were significantly higher compared to the normal un-transfected cells or mock cells.
This prevention of mtDNA damage was accompanied by amelioration of glucose-induced increases in mRNA levels of DNA repair enzyme OGG1 (Figure 2) along with its decreased nuclear accumulation in the cells transfected with MnSOD and decreased superoxide radicals as depicted by Mito-Tracker staining (Figure 3). In addition, supplementation with MnTBAP during high glucose incubation or over-expression of MnSOD also prevented any decrease in mitochondrial GSH levels and increase in its membrane permeability (Figure 4).
Over-expression of MnSOD had a beneficial effect on the functional consequence of mtDNA damage: gene expressions of ND1, ND4 and cytochrome b were significantly higher in the MnSOD-transfected cells exposed to high glucose compared to normal or mock cells exposed to high glucose (Figure 5). In addition, cytochrome b mRNA values obtained from MnSOD-transfected cells incubated in 5 mM glucose were significantly higher than those from the un-transfected control cells in 5 mM glucose. The reason for such increase is not clear and the possibility that due to inherent oxidative stress cytochrome b of complex III is ‘somewhat’ compromised cannot be ruled out. Although the magnitude of this inherent oxidative stress appears to be significantly lower than the one experienced in high glucose, over-expression of MnSOD overcomes that by increasing cytochrome b gene expression.
Discussion
Diabetes induces mitochondrial damage in the retina and its capillary cells and mitochondrial dysfunction is considered to play a significant role in the development of retinopathy [4,5]. Regulation of superoxide radicals by MnTBAP or by MnSOD over-expression prevents retinal endothelial cells from undergoing accelerated apoptosis that precedes the histopathology of diabetic retinopathy [2–7]. Here we show that the cells of the retinal microvasculature that are the site of histopathology characteristic of diabetic retinopathy respond to a high glucose environment with increased mtDNA damage and aberrant repair machinery. This is followed by subsequent decreases in mitochondrial-encoded genes critical to the formation of complexes I and III of the electron transport chain. MnSOD plays a significant role in preventing damage to mtDNA; inhibition of accumulation of superoxide radicals (by MnTBAP or over-expression of MnSOD) ameliorates glucose-induced mtDNA damage and reductions in the mitochondrial-encoded genes.
The close proximity to the electron transport chain, less tightly packaging by DNA transcription factors and chaperones and lack of histone make mtDNA more vulnerable to damage from insults generated by the electron transport chain than nDNA [14]. Mitochondrial superoxide radicals are considered to act as a unifying mechanism promoting various reactions leading to the development of diabetic complications [26]. Due to dysfunctional mitochondria, ROS production is further increased and this exacerbates the oxidative stress in mitochondria [27]. In animal models of diabetic complications others have shown a major role of oxidatively damaged mtDNA in the pathogenesis of diabetic nephropathy [28]. Glucose-induced ROS production is associated with altered mitochondrial morphology [29]. Here, our data show that over-production of free radicals in retinal endothelial cells is directly associated with mtDNA damage; prevention of production of superoxide radicals or pharmacologic means, in addition to ameliorating glucose-induced increased superoxide radicals and mitochondrial dysfunction, also inhibits mtDNA damage and protects its functional consequences. This implies that mtDNA damage of retinal capillary cells could be further contributing to increased generation of ROS, which would exacerbate their oxidative damage.
In order to maintain constant homeostasis mitochondria has efficient repair machinery; mammalian mitochondria are well equipped with a fully functional BER system consisting of various glycosylases including OGG1, uracil DNA glycosylase and thymine glycol DNA glycosylase [19,30]. 8-hydroxydeoxyguanosine is one of the most prevalent products of the oxidative DNA damage and it is accumulated in the mtDNA at higher concentrations than in nDNA [22]. We have shown that the levels of 8-hydroxyde-oxyguanosine are elevated in the retina in diabetes [23]. For the repair of oxoguanine lesions in DNA, OGG1 is the main DNA glycosylase and mitochondria are more dependent on OGG1 for the removal of 8-hydroxydeoxyguanosine than the nucleus [19,31]. The mtDNA repair enzymes, however, are encoded by the same genes as their nuclear counterparts [32]. These are transported to the mitochondria via a complex transport process that involves mitochondrial potential [33]. We show that high glucose exposure, in addition to damaging mtDNA, also affects the OGG1 repair machinery; although the cell tries to increase the production of OGG1 to overcome the mtDNA damage, its translocation from the nucleus is impaired, contributing to an over-compromised repair system in the mitochondria. In support, others have reported promotion of oxidative damage by over-expression of OGG1 [34]. However, when superoxide radicals are quenched, glucose-induced increase in membrane permeability is ameliorated along with the gene expression and subcellular distribution of OGG1, suggesting that OGG1 is in the direct control of superoxide in maintaining the homeostasis.
Mitochondrial GSH has been considered to regulate mtDNA damage and recovery and is critical in protecting mtDNA from oxidative injury [35]. Here we show that glucose exposure decreases the levels of mitochondrial GSH and MnSOD abrogates such reductions. This implies that, in addition to mtDNA damage and compromised repair machinery, glucose exposure diminishes the levels of the intra-mitochondrial antioxidant, GSH, and decreased mitochondrial GSH levels further exacerbate mtDNA damage. The plausible reason for decreased mitochondrial GSH could be its increased oxidation or impaired synthesis. In support, we have shown that in diabetes the ratio of GSH/GSSG is decreased in the retinal cytosol and the activity of gamma-glutamyl transpeptidase, an enzyme important in the synthesis and degradation of glutathione, becomes sub-normal [36,37].
Mitochondrial DNA encodes only 13 proteins and these are mainly from the electron transport chain complexes of the mitochondrial inner membrane [38]. Abnormalities in mtDNA encoded genes are related with many pathological conditions, including dilated cardiomyopathies [39]. Recent studies have shown that different mtDNA haplogroups are associated with the subtle differences in oxidative phosphorylation systems and the presence of mitochondrial haplogroup T is significantly associated with the presence of coronary artery disease and retinopathy in diabetic patients [40]. We show that high glucose induces reductions in the gene expressions of mtDNA encoded proteins ND1 and ND4 of complex I and cytochrome b of complex III and MnSOD protects them from such insult. These results strongly imply that, due to high glucose-induced damage of mtDNA, the electron transport system is compromised and regulation of superoxide radicals directly influences the electron transport system. The possibility that high glucose exposure of retinal endothelial cells first damages mtDNA resulting in increased production of superoxide radicals, as suggested by others [9], however, cannot be ruled out.
We recognize that the effects of MnTBAP observed in our study could be via scavenging peroxynitrite [41], but the results presented in the present study were confirmed using cells that were genetically manipulated for MnSOD and strongly support the role of superoxide in regulation of mtDNA homeostasis.
In conclusion, we have provided direct evidence that in hyperglycaemia the mitochondria of the retinal capillary cells, the cells that are the target of pathology of diabetic retinopathy, have damaged DNA and impaired repair systems and the proteins of electron transport chain complexes are also subnormal, contributing to a continuous vicious cycle of increased oxidative damage. MnSOD provides protection from such damage and the possible mechanism by which MnSOD ameliorates the development of diabetic retinopathy includes the protection of mtDNA from oxidative damage and its related consequences. Thus, prevention of mtDNA by MnSOD mimic could have potential in ameliorating the development of retinopathy, the sight-threatening disease that diabetic patients fear the most.
Acknowledgments
Technical assistance of Mamta Kanwar is greatly appreciated.
Footnotes
Declaration of interest: This study was supported in part by grants from the National Institutes of Health, Juvenile Diabetes Research Foundation, Thomas Foundation, and Research to Prevent Blindness. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996;97:2883–2890. doi: 10.1172/JCI118746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kowluru RA, Odenbach S. Effect of long-term administration of alpha lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes. 2004;53:3233–3238. doi: 10.2337/diabetes.53.12.3233. [DOI] [PubMed] [Google Scholar]
- 3.Kern TS, Tang J, Mizutani M, Kowluru R, Nagraj R, Lorenzi M. Response of capillary cell death to aminoguanidine predicts the development of retinopathy: comparison of diabetes and galactosemia. Invest Ophthalmol Vis Sci. 2000;41:3972–3978. [PubMed] [Google Scholar]
- 4.Kowluru RA, Abbas SN. Diabetes-induced mitochondrial dysfunction in the retina. Inves Ophthalmol Vis Sci. 2003;44:5327–5334. doi: 10.1167/iovs.03-0353. [DOI] [PubMed] [Google Scholar]
- 5.Kowluru RA. Diabetic retinopathy: mitochondrial dysfunction and retinal capillary cell death. Antiox Redox Signal. 2005;7:1581–1587. doi: 10.1089/ars.2005.7.1581. [DOI] [PubMed] [Google Scholar]
- 6.Kowluru RA, Atasi L, Ho YS. Role of mitochondrial superoxide dismutase in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2006;47:1594–1599. doi: 10.1167/iovs.05-1276. [DOI] [PubMed] [Google Scholar]
- 7.Kanwar M, Chan PS, Kern TS, Kowluru RA. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci. 2007;48:3805–3811. doi: 10.1167/iovs.06-1280. [DOI] [PubMed] [Google Scholar]
- 8.Kowluru RA, Chan PS. Metabolic memory in diabetes—from in vitro oddity to in vivo problem: role of apoptosis. Brain Res Bull. 2009 May;20 doi: 10.1016/j.brainresbull.2009.05.006. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie L, Zhu X, Hu Y, Li T, Gao Y, Shi Y, Tang S. Mitochondrial DNA oxidative damage triggering mitochondrial dysfunction and apoptosis in high glucose-induced HRECs. Invest Ophthalmol Vis Sci. 2008;49:4203–4209. doi: 10.1167/iovs.07-1364. [DOI] [PubMed] [Google Scholar]
- 10.Gerhardinger C, Dagher Z, Sebastiani P, Park YS, Lorenzi M. The transforming growth factor-beta pathway is a common target of drugs that prevent experimental diabetic retinopathy. Diabetes. 2009;58:1659–1667. doi: 10.2337/db08-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kowluru RA, Kern TS, Engerman RL. Abnormalities of retinal metabolism in diabetes or experimental galactosemia. IV. Antioxidant defense system. Free Radic Biol Med. 1997;22:587–592. doi: 10.1016/s0891-5849(96)00347-4. [DOI] [PubMed] [Google Scholar]
- 12.Kowluru RA, Kowluru V, Ho YS, Xiong Y. Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Radic Biol Med. 2006;41:1191–1196. doi: 10.1016/j.freeradbiomed.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Bohr VA. Repair of oxidative DNA damage in nuclear and mitochondrial DNA, and some changes with aging in mammalian cells. Free Radic Biol Med. 2002;32:804–812. doi: 10.1016/s0891-5849(02)00787-6. [DOI] [PubMed] [Google Scholar]
- 14.Chen XJ, Butow RA. The organization and inheritance of the mitochondrial genome. Nat Rev Genet. 2005;6:815–825. doi: 10.1038/nrg1708. [DOI] [PubMed] [Google Scholar]
- 15.Kucej M, Kucejova B, Subramanian R, Chen XJ, Butow RA. Mitochondrial nucleoids undergo remodeling in response to metabolic cues. J Cell Sci. 2008;21:1861–1868. doi: 10.1242/jcs.028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stuart JA, Brown MF. Mitochondrial DNA maintenance and bioenergetics. Biochim Biophys Acta. 2006;1757:79–89. doi: 10.1016/j.bbabio.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Weissman L, de Souza-Pinto NC, Stevnsner T, Bohr VA. DNA repair, mitochondria, and neurodegeneration. Neuroscience. 2007;145:1318–1329. doi: 10.1016/j.neuroscience.2006.08.061. [DOI] [PubMed] [Google Scholar]
- 18.Hegde ML, Hazra TK, Mitra S. Early steps in the DNA base excision/single-strand interruption repair pathway in mammalian cells. Cell Res. 2008;18:27–47. doi: 10.1038/cr.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukae J, Mizuno Y, Hattori N. Mitochondrial dysfunction in Parkinson’s disease. Mitochondrion. 2007;7:58–62. doi: 10.1016/j.mito.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Kowluru RA, Koppolu P. Diabetes-induced activation of caspase-3 in retina: effect of antioxidant therapy. Free Radic Res. 2002;36:993–999. doi: 10.1080/1071576021000006572. [DOI] [PubMed] [Google Scholar]
- 21.Ayala-Torres S, Chen Y, Svoboda T, Rosenblatt J, Van Houten B. Analysis of gene-specific DNA damage and repair using quantitative polymerase chain reaction. Methods. 2000;22:135–147. doi: 10.1006/meth.2000.1054. [DOI] [PubMed] [Google Scholar]
- 22.Wang AL, Lukas TJ, Yuan M, Neufeld AH. Age-related increase in mitochondrial DNA damage and loss of DNA repair capacity in the neural retina. Neurobiol Aging. 2008 December;10 doi: 10.1016/j.neurobiolaging.2008.10.019. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Kowluru RA, Menon B, Gierhart D. Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rat. Inves Ophthalmol Vis Sci. 2008;49:1645–1651. doi: 10.1167/iovs.07-0764. [DOI] [PubMed] [Google Scholar]
- 24.Busik JV, Mohr S, Grant MB. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes. 2008;57:1952–1965. doi: 10.2337/db07-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poot M, Zhang YZ, Kramer JA, Well KS, Jones LJ, Hanzel DK, Lugade AG, Singer VL, Haugland RP. Analysis of mitochondrial morphology and function with novel fixable fluorescent stains. J Histochem Cytochem. 1996;44:1363–1372. doi: 10.1177/44.12.8985128. [DOI] [PubMed] [Google Scholar]
- 26.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 27.Rachek LI, Musiyenko SI, LeDoux SP, Wilson GL. Palmitate induced mitochondrial deoxyribonucleic acid damage and apoptosis in l6 rat skeletal muscle cells. Endocrinology. 2007;148:293–299. doi: 10.1210/en.2006-0998. [DOI] [PubMed] [Google Scholar]
- 28.Kakimoto M, Inoguchi T, Sonta T, Yu HY, Imamura M, Etoh T, Hashimoto T, Nawata H. Accumulation of 8-hydroxy-2′-deoxyguanosine and mitochondrial DNA deletion in kidney of diabetic rats. Diabetes. 2002;51:1588–1595. doi: 10.2337/diabetes.51.5.1588. [DOI] [PubMed] [Google Scholar]
- 29.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang D, Hamasaki N. Maintenance of mitochondrial DNA integrity: repair and degradation. Curr Genet. 2002;41:311–322. doi: 10.1007/s00294-002-0312-0. [DOI] [PubMed] [Google Scholar]
- 31.de Souza-Pinto NC, Eide L, Hogue BA, Thybo T, Stevnsner T, Seeberg E, Stevnser T, Seeberg E, Klungland A. Repair of 8-oxodeoxyguanosine lesions in mitochondrial DNA depends on the oxoguanine DNA glycosylase (OGG1) gene and 8-oxoguanine accumulates in the mitochondrial dna of OGG1-defective mice. Cancer Res. 2002;61:5378–5381. [PubMed] [Google Scholar]
- 32.Page MM, Stuart JA. In vitro measurement of DNA base excision repair in isolated mitochondria. Methods Mol Biol. 2009;554:213–231. doi: 10.1007/978-1-59745-521-3_14. [DOI] [PubMed] [Google Scholar]
- 33.Bolender N, Sickmann A, Wagner R, Meisinger C, Pfanner N. Multiple pathways for sorting mitochondrial precursor proteins. EMBO J. 2008;9:42–49. doi: 10.1038/sj.embor.7401126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Mizumachi T, Carcel-Trullols J, Li L, Naito A, Spencer HJ, Spring PM, Smoller BR, Watson AJ, Margison GP. Targeting human 8-oxoguanine DNA glycosylase (hOGG1) to mitochondria enhances cisplatin cytotoxicity in hepatoma cells. Carcinogenesis. 2007;28:1629–1637. doi: 10.1093/carcin/bgm072. [DOI] [PubMed] [Google Scholar]
- 35.Circu ML, Moyer MP, Harrison L, Aw TY. Contribution of glutathione status to oxidant-induced mitochondrial DNA damage in colonic epithelial cells. Free Radic Biol Med. 2009;47:1190–1198. doi: 10.1016/j.freeradbiomed.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kern TS, Kowluru RA, Engerman RL. Abnormalities of retinal metabolism in diabetes or galactosemia. I. ATPases and glutathione. Inves Ophthal Vis Sci. 1994;35:2962–2967. [PubMed] [Google Scholar]
- 37.Kowluru RA, Kern TS, Engerman RL. Abnormalities of retinal metabolism in diabetes and galactosemia. II. Comparison of gamma-glutamyl transpeptidase in retina and cerebral cortex and effects of antioxidant therapy. Curr Eye Res. 1994;13:891–896. doi: 10.3109/02713689409015092. [DOI] [PubMed] [Google Scholar]
- 38.Szibor M, Holtz J. Mitochondrial ageing. Basic Res Cardiol. 2003;98:210–218. doi: 10.1007/s00395-003-0421-z. [DOI] [PubMed] [Google Scholar]
- 39.Monsalve M, Borniquel S, Valle I, Lamas S. Mitochondrial dysfunction in human pathologies. Front Biosci. 2007;12:1131–1153. doi: 10.2741/2132. [DOI] [PubMed] [Google Scholar]
- 40.Kofler B, Mueller EE, Eder W, Stanger O, Maier R, Weger M, Haas A, Winkler R, Schmut O, Paulweber B, Iglseder B, Renner W, Wiesbaur M, Aigner I, Santic D, Zimmerman A, Sperl W. Mitochondrial DNA haplogroup T is associated with coronary artery disease and diabetic retinopathy: a case control study. BMC Med Genet. 2009;10:35. doi: 10.1186/1471-2350-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Batinić-Haberle I, Cuzzocrea S, Rebouças JS, Ferrer-Sueta G, Mazzon E, Di Paola R, Radi R, Spasojević I, Benov L, Salvemini D. Pure MnTBAP selectively scavenges peroxynitrite over superoxide: comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two models of oxidative stress injury, an SOD-specific Escherichia coli model and carrageenan-induced pleurisy. Free Radic Biol Med. 2009;46:192–201. doi: 10.1016/j.freeradbiomed.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]