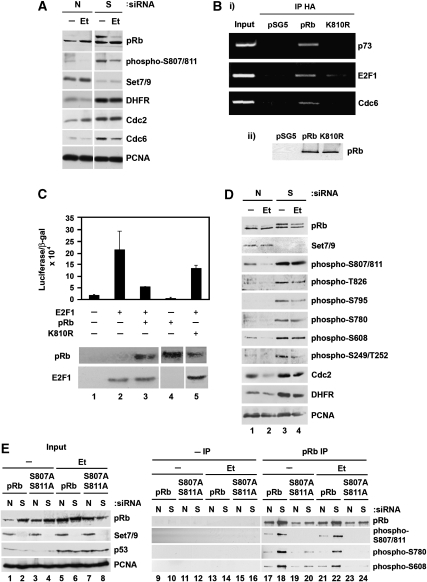
Figure 5.
Consequences of pRb methylation on cell cycle regulation. (A) U2OS cells were transfected with 20 nM Set7/9 (S) or non-targeting (N) siRNA for 72 h. Cells were also treated with 10 μM etoposide (Et) for the last 16 h where appropriate. Cell extracts were then prepared and immunoblotted with the indicated antibodies. (B) (i) SAOS2 cells were transfected with empty vector (pSG5), HA-pRb or HA-pRb K810R (4 μg) as indicated. After 48 h, cells were cross-linked in formaldehyde and chromatin immunoprecipitation samples were prepared. Immunoprecipitation was performed using anti-HA antibody and analysed by PCR. (ii) Input levels of transfected HA-pRb and HA-pRb K810R were detected by immunoblotting with anti-HA antibody (pRb). (C) SAOS2 cells were transfected with HA-pRb or HA-pRb K810R (2 μg), together with HA-E2F-1 (300 ng), the pCycE-luc reporter plasmid (300 ng) and pCMV-βgal (300 ng) as the internal control. The relative reporter activity (luciferase/β-galactosidase) is indicated with standard deviation as shown. Lower panel shows the levels of pRb and E2F-1 detected by immunoblotting with anti-HA antibody. (D) U2OS cells were transfected with 20 nM Set7/9 (S) or non-targeting (N) siRNA for 72 h. Cells were also treated with 10 μM etoposide (Et) for the last 16 h where appropriate. Cell extracts were then prepared and immunoblotted with the indicated antibodies. (E) U2OS cells were transfected with 20 nM Set7/9 (S) or non-targeting (N) siRNA. After 24 h, cells were transfected with expression vectors for HA-pRb or HA-pRb S807A/S811A (4 μg) and left for further 48 h. Cells were also treated with 10 μM etoposide (Et) for the last 16 h where appropriate. Cell extracts were immunoprecipitated with anti-HA (pRb IP) or non-specific (–IP) antibody. Immunoprecipitated pRb was immunoblotted with anti-HA (pRb), anti-phospho-S807/811, anti-phospho-S780 or anti-phospho-S608 antibody where indicated. Quantitation of the results is presented in Supplementary Figure S1F.