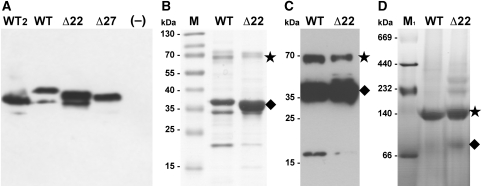
Figure 6.
Biochemical characterization of NhaP1. (A) Western blot of everted vesicles used in the activity assay. 30 μg total protein was loaded per lane and detected with an antibody against the C-terminal helix of NhaP1 coupled to horseradish peroxidase. (B) Ni-NTA-affinity purified WT and Δ22 protein (5 μg) separated on a 12% gel and stained with Coomassie brilliant blue. (C) Western blot of Ni-NTA-affinity purified WT and Δ22 protein (5 μg) transferred to a PVDF membrane, immunostained with anti-myc antibody coupled to horseradish peroxidase. (D) Blue-native gel electrophoresis of WT (30 μg) and Δ22 (20 μg). Samples were run on 4–16% Bis/Tris native-PAGE and destained. The NhaP1 dimer is indicated with an asterisk, the monomer by a diamond. WT: NhaP1wt-Myc-His; WT2: NhaP1wt-His; Δ22: NhaP1Δ22-Myc-His; Δ27: NhaP1Δ27-Myc-His; –: untransformed KNabc cells; M: prestained protein marker (Fermentas); M1: HMW protein mix for native electrophoresis (GE Healthcare).