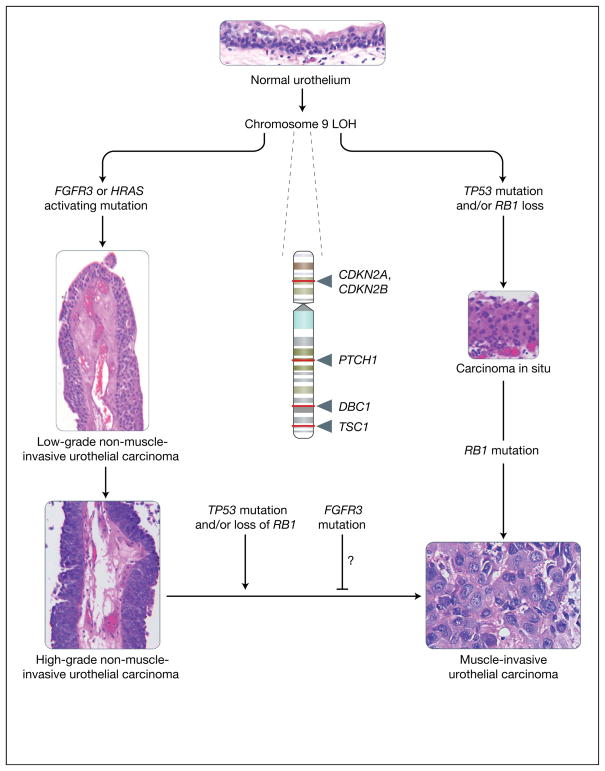
Figure 1. Molecular lineage of non-muscle-invasive urothelial cancer versus muscle-invasive urothelial cancer.
The figure summarises the major molecular alterations that are responsible for the onset of non-muscle-invasive urothelial carcinomas (NMIUCs) and muscle invasive urothelial carcinomas (MIUCs), along with providing representative haematoxylin and eosin micrographs of these disease states. Starting from a normal urothelium five to seven cells thick (top), loss of heterozygosity (LOH) of chromosome 9 (arrows indicate relative sites of potential tumour suppressor gene loss; names of genes are given) has been associated with the majority of urothelial cancers. This is often coincident with a mutation in either FGFR3 or HRAS, which have been implicated in the genesis of NMIUCs (low- and high-grade papillary NMIUCs indicated on the left show examples of the increasing degree of architectural disorder and increasing cytologic atypia). FGFR3 and HRAS mutations are usually not present within the same cancer. By contrast, from a molecular standpoint, the MIUC class of tumours is defined by mutations in the tumour suppressor genes TP53 and RB1, which frequently coincide in the same tumours. Generally, MIUCs are thought to arise de novo from carcinoma in situ (shown on the right). Histologically, marked cytologic atypia (high ratio of nuclei to cytoplasm, mitoses, and total loss of tissue architecture) are present (bottom right) in a context of observation of definitive invasion of the muscularis propria of the bladder, in contrast to NMIUCs. As a class, NMIUCs have a high tendency to recur and rarely progress to a higher-grade carcinoma or MIUC. When this progression does occur, it is usually coincident with loss of function of the tumour suppressors TP53 or RB1. Some studies have shown that the presence of activating FGFR3 mutations correlate to a reduced frequency of progression to a higher-grade/stage cancer, but these events have not been definitively shown to be causal. CDKN2A, cyclin-dependent kinase inhibitor 2A (also known as P16); CDKN2B, cyclin-dependent kinase inhibitor 2B (also known as P15); DBC1, deleted in bladder cancer 1; FGFR3, fibroblast growth factor receptor 3; HRAS, Harvey rat sarcoma viral gene homologue; PTCH1, patched homologue 1; RB1, retinoblastoma tumour suppressor; TP53, p53 tumour suppressor; TSC1, tuberous sclerosis 1 gene. [Images: S.C. Smith. Originally photographed at 40× (NMIUCs) and 60× (MIUC, carcinoma in situ and urothelium)].