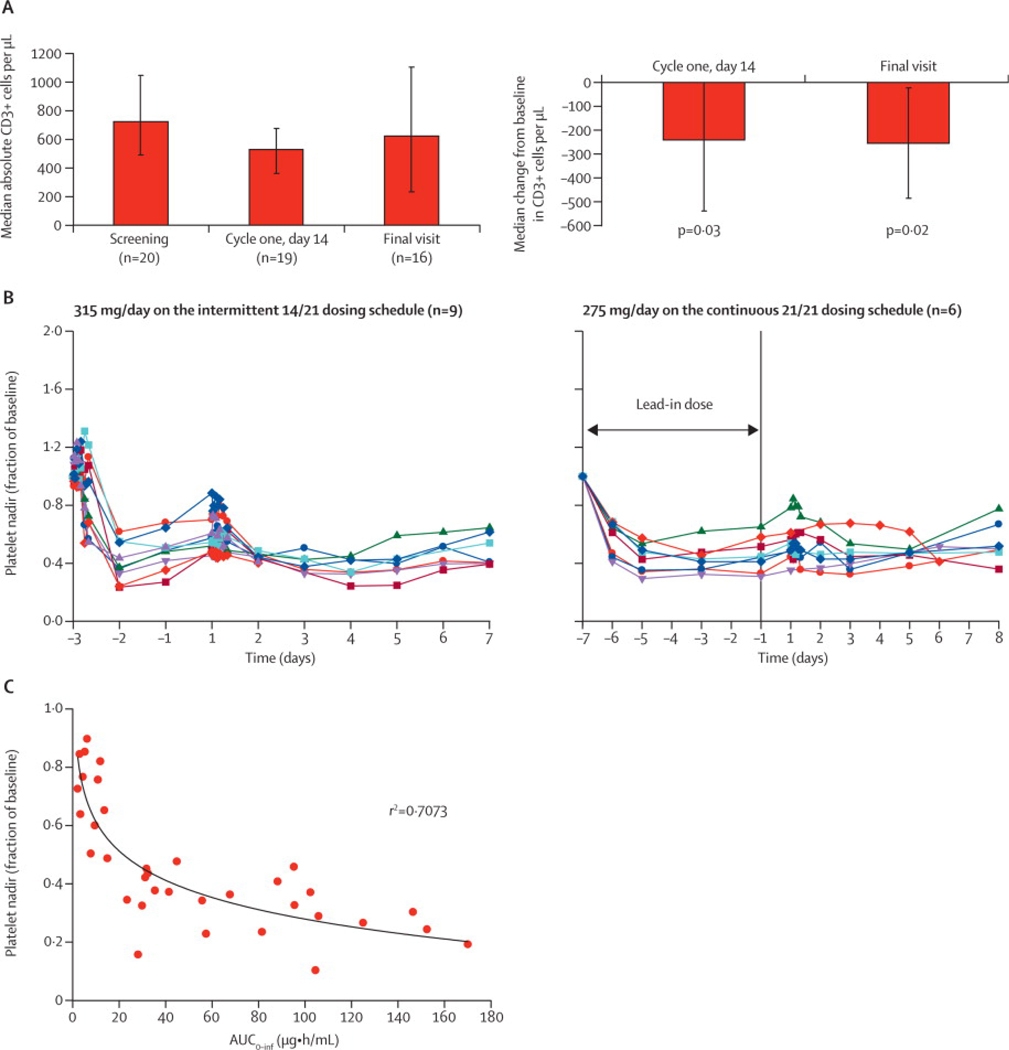
Figure 3. Pharmacodynamic Effects of Navitoclax.
(a) Bar graph of median absolute circulating CD3 cells/µl at baseline (n=20 patients), day 14 of cycle 1 (n=19 patients) and the final visit (n=16 patients) at the end of navitoclax treatment, and the median change on day 14 of cycle 1 and final visit. Patients received at least 200 mg navitoclax for at least two cycles on either the 14/21 or 21/21day schedule. The mean (range) time between baseline and final visit was 89 (29–332) days and the mean (range) daily drug exposure was 255 (137–388) mg of navitoclax. P-value was calculated using a Sign-test because the data were skewed. (b) Line graph of platelet nadirs over time at 315 mg on the 14/21-day schedule (n=9 patients) (left panel) and at 275 mg on the 21/21-day schedule (n=6 patients) (right panel). (c) Platelet nadir versus navitoclax area under the curve at multiple dose levels (n=35 patients). R-value was calculated by using SigmaPlot 9.0.