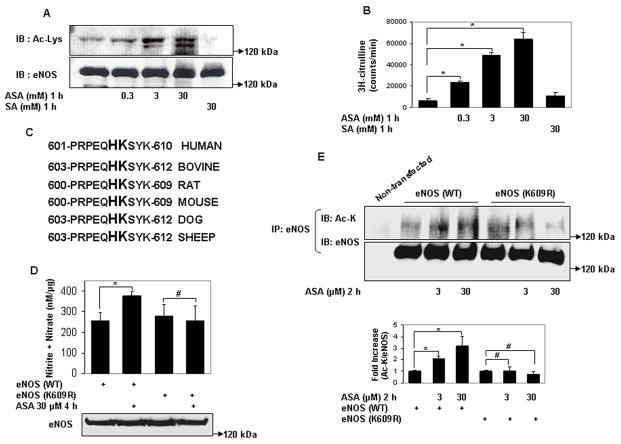
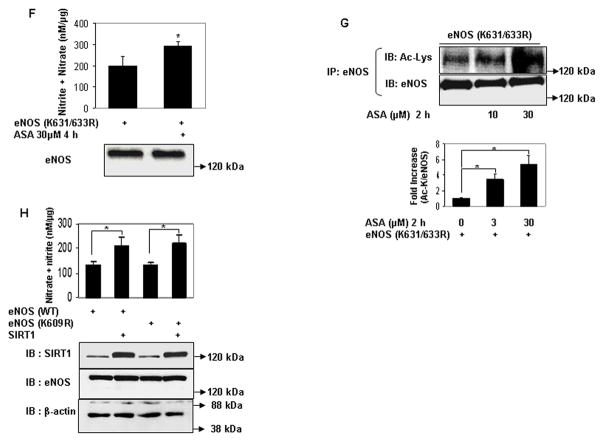
Figure 2.
Aspirin directly acetylates eNOS on lysine residues, and acetylation of lysine 609 in eNOS mediates aspirin stimulated eNOS NO production. A and B, Lysine acetylation and activation of eNOS by aspirin in vitro. In vitro concentration dependent (A) lysine acetylation (Ac-Lys, Ac-K), and (B) enzymatic activity of recombinant eNOS by aspirin. C, Conservation of the tandem histidine-lysine pair consisting of lysine 609 (in bovine eNOS) in the calmodulin auto-inhibitory domains of eNOS of different species. D through G, Aspirin promotes eNOS-derived NO by specifically targeting lysine 609 in bovine eNOS. D and F, NO (nitrite + nitrate) measured in conditioned media, and E and G, lysine acetylation (Ac-Lys, Ac-K) of eNOS in lysates of HEK 293 cells expressing ectopic wildtype eNOS (eNOS WT), eNOS with lysine 609 mutated to nonacetylatable arginine (eNOS K609R or eNOS) nonacetylatable on lysines 631 and 633 (eNOS K631/633R) and treated with a low concentration of ASA. H, SIRT1 does not increase NO through deacetylation of lysine 609. NO (nitrite + nitrate) in conditioned media of HEK 293 cells expressing ectopic eNOS (WT) and eNOS (K609R), with and without SIRT1 overexpression. Representative immunoblots are shown with accompanying analysis by densitometry of Ac-K/total eNOS relative to no aspirin from independent blots. *P<0.05 and #P=NS (n=3 to 5).