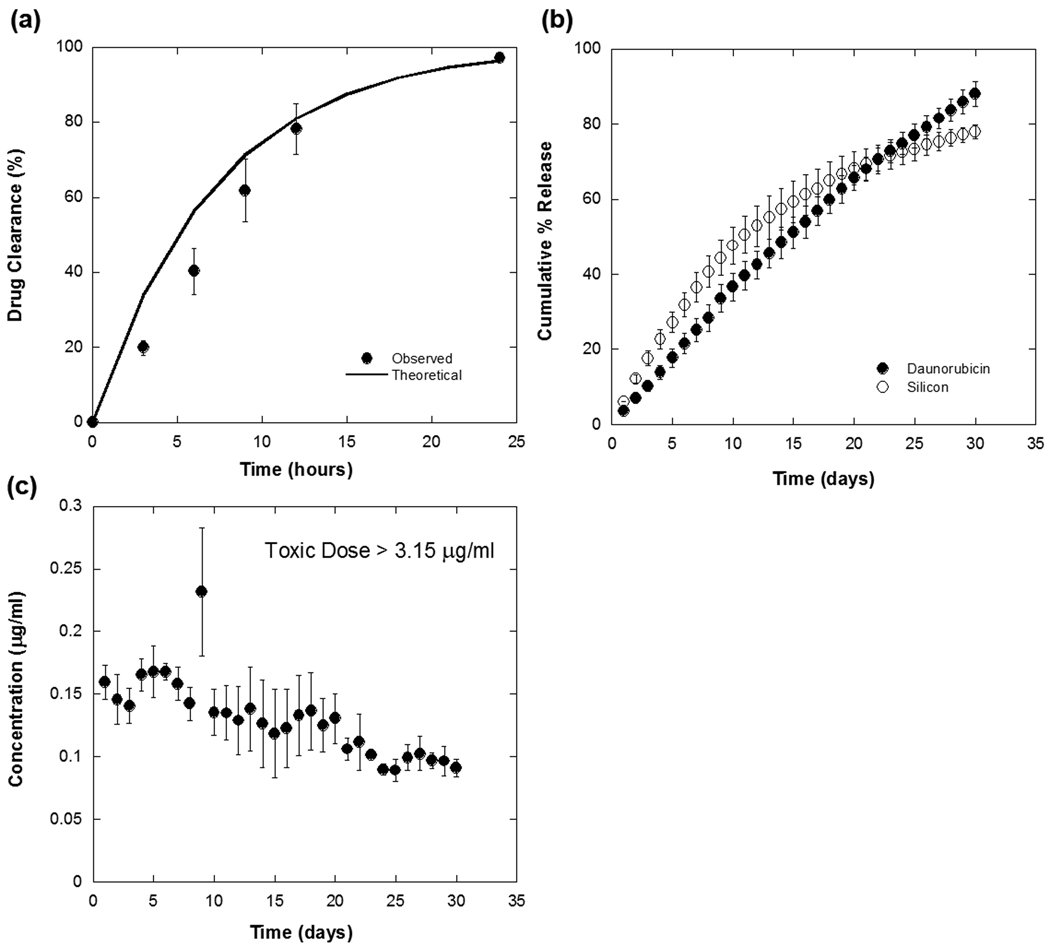
Figure 2.
In vitro release of daunorubicin covalently attached to porous Si microparticles. (a) Control experiment, showing the rapid clearance (t1/2 = 5 h) of an aliquot of free daunorubicin injected into the flow chamber at time t = 0 and then eluted with a continuous flow of 450 µL/h of pure PBS solution. The solid line shown is the calculated % cleared as a function of time in hours, based on the elution rate and chamber volume. (b) Percent of daunorubicin (solid circles, “drug”) and silicon (open circles, “Si”) released from porous Si microparticles into the effluent stream under the same flow conditions as in (a). Note the time axis here is in units of days. The effective t1/2 for clearance of the covalently attached drug from the chamber is increased from 5 h (for free drug) to 15 d. Soluble silicon species (orthosilicate ions) and soluble daunorubicin quantified by ICP-OES and fluorimetry measurements, respectively. (c) Steady-state concentration of daunorubicin in the chamber as a function of time for the experiment described in (b). A therapeutic level of drug is maintained for > 30 d.