TABLE 1.
Oxidation of Electron-rich and Electron-neutral Potassium Aryltrifluoroboratesa
 | ||||
|---|---|---|---|---|
| entry | substrate | product | isolated yield (%) | |
| 1 |
|
|
1a | 96b |
| 2 |
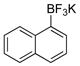
|
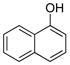
|
1b | 99 |
| 3 |
|
|
1c | 97 |
| 4 |
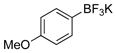
|
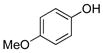
|
1d | 98 |
| 5 |
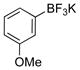
|
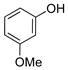
|
1e | 93 |
| 6 |
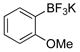
|
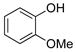
|
1f | 97 |
| 7 |
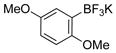
|
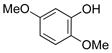
|
1g | 95 |
| 8 |
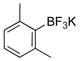
|
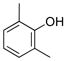
|
1h | 94 |
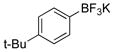
| 9 |
|
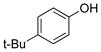
|
1i | 99 |
| 10 |
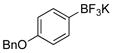
|
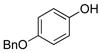
|
1j | 97 |
All reactions were carried out using 1 mmol of aryltrifluoroborate and Oxone (5 mL, 0.2 M in H2O) in 5 mL of acetone for 2 min at rt.
55 mmol scale.
