Abstract
The mitochondrial permeability transition (mPT) is considered to be a major cause of cell death under a variety of pathophysiological conditions of the central nervous system (CNS) and other organs. Pharmacological inhibition or genetic knockout of the matrix protein cyclophilin D (CypD) prevents mPT and cell degeneration in several models of brain injury. If these findings in animal models are translatable to human disease, pharmacological inhibition of mPT offers a promising therapeutic target. The objective of this study was to validate the presence of a CypD-sensitive mPT in adult human brain and liver mitochondria. In order to perform functional characterization of human mitochondria, fresh tissue samples were obtained during hemorrhage or tumor surgery and mitochondria were rapidly isolated. Mitochondrial calcium retention capacity, a quantitative assay for mPT, was significantly increased by the CypD inhibitor cyclosporin A in both human brain and liver mitochondria, whereas thiol-reactive compounds and oxidants sensitized mitochondria to calcium-induced mPT. Brain mitochondria underwent swelling upon calcium overload, which was reversible upon calcium removal. To further explore mPT of human mitochondria, liver mitochondria were demonstrated to exhibit several classical features of the mPT phenomenon, such as calcium-induced loss of membrane potential and respiratory coupling, as well as release of the pro-apoptotic protein cytochrome c. We concluded that adult viable human brain and liver mitochondria possess an active CypD-sensitive mPT. Our findings support the rationale of CypD and mPT inhibition as pharmacological targets in acute and chronic neurodegeneration.
Key words: ischemia, oxidative stress, mitochondria, traumatic brain injury, traumatic spinal cord injury
Introduction
Activation of mitochondrial permeability transition (mPT) is considered to be a major cause of cell death under a variety of pathophysiological conditions, including ischemia/reperfusion injury, neurodegenerative disease, traumatic brain injury (TBI), muscular dystrophy, and drug toxicity (Bernardi et al., 2006; Halestrap and Pasdois, 2009; Kroemer et al., 2007; Liu and Murphy, 2009; Mbye et al., 2009; Millay et al., 2008; Nicholls, 2009; Russmann et al., 2009). Provided that findings in animal models can be translated to human disease, pharmacological inhibition of mPT offers a promising therapeutic target for the treatment of these disorders (Baines, 2010; Cook et al., 2009; Morota et al., 2009; Waldmeier et al., 2003).
The mPT is defined as a sudden increase in inner mitochondrial membrane permeability causing loss of ion homeostasis and the proton motive force required for ATP synthesis. The matrix protein cyclophilin D (CypD) is a peptidylprolyl cis-trans isomerase that regulates mPT and facilitates its activation by calcium. Animals lacking CypD display increased resistance to ischemic insults, muscular dystrophies, multiple sclerosis, amyotrophic lateral sclerosis (ALS), and Alzheimer's disease (Baines et al., 2005; Du et al., 2008; Forte et al., 2007; Martin et al., 2009; Nakagawa et al., 2005; Schinzel et al., 2005), and the CypD inhibitor cyclosporin A (CsA) and its analogs have displayed neuroprotective effects in several animal models of acute neurological damage and chronic neurodegenerative disease. CsA treatment has also demonstrated promising results in initial clinical trials of TBI (Empey et al., 2006; Hatton et al., 2008; Mazzeo et al., 2008), as well as myocardial reperfusion injury (Mewton et al., 2010; Piot et al., 2008). Preserving the integrity of mitochondrial membranes through inhibition of mPT has been put forward as the central mechanism for the neuroprotective and cardioprotective effects of CsA, even though the drug has several pharmacological targets. Further, it has not been established whether cellular calcium overload and oxidative stress can trigger the mPT phenomenon in adult human mitochondria similarly to that described in mitochondria derived from animal tissues. It has also been suggested that CypD is downregulated in neurons during development, which would decrease the sensitivity of the mPT to calcium, and prohibit the use of CypD as a pharmacological target in disorders of the adult central nervous system (CNS) (Eliseev et al., 2007).
The purpose of this study was to determine whether a CypD-sensitive mPT exists in adult human brain and liver mitochondria, and thus constitutes a relevant pharmacological target in diseases for which mPT has been implicated in the pathogenesis. Further, if present, the objective was to evaluate whether human mPT displays analogous functional characteristics and is modulated by endogenous regulators and oxidants similarly to mitochondria from animal tissues.
Methods
Tissue samples
In order to obtain fresh human tissue for functional mitochondrial analyses, brain samples were collected from five patients undergoing neurosurgery, and liver tissue from seven patients undergoing liver resection (see Table 1 for patient demographics). Tissue that would otherwise have been discarded was transferred into ice-cold isolation buffer and rapidly prepared for mitochondrial isolation. Only the morphologically normal parenchyma surrounding the resected tumors was used. The human study was approved by the Ethical Committee of Hachioji Medical Center, Tokyo Medical University, permit number 12-01, and complied with the World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects, and the EU Convention for the Protection of Human Rights and Dignity of the Human Being with Regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine.
Table 1.
Patient Demographics, Indications for Surgery, and Origin of Tissue Samples
| Brain (B) or liver (L) sample | Gender | Age (y) | Indication for surgery | Origin of tissue |
|---|---|---|---|---|
| B1 | Male | 45 | Arteriovenous malformation | Right temporal cortex |
| B2 | Male | 72 | Intracerebral hemorrhage | Caudate putamen |
| B3 | Male | 42 | Subarachnoid hemorrhage | Right frontal cortex |
| B4 | Male | 81 | Intracerebral hemorrhage | Right frontal cortex |
| B5 | Male | 62 | Glioma | Right temporal cortex |
| L1 | Male | 58 | Metastatic colon cancer | Quadrate lobe |
| L2 | Male | 60 | Metastatic colon cancer | Quadrate lobe |
| L3 | Male | 65 | Metastatic colon cancer | Left lateral segment |
| L4 | Male | 57 | Metastatic colon cancer | Left lateral segment |
| L5 | Male | 56 | Bile duct cancer | Quadrate lobe |
| L6 | Female | 80 | Metastatic liver carcinoma | Quadrate lobe |
| L7 | Female | 74 | Metastatic liver carcinoma | Quadrate lobe |
Mitochondrial isolation
Isolation of human brain mitochondria was achieved using a discontinuous Percoll gradient according to the method of Sims and Anderson (Sims and Anderson, 2008), with slight modification as previously described (Hansson et al., 2008), and the three density gradient layers consisted of 12%, 19%, and 40% Percoll, respectively. Liver mitochondria were isolated using differential centrifugation including a 19% Percoll step (Hansson et al., 2008).
Mitochondrial permeability transition assays
A Perkin-Elmer luminescence spectrometer (LS-55B; Emeryville, CA) with a temperature-controlled cuvette holder was used for all fluorescence and light scattering experiments. De-energized experiments were performed at 28°C in a 150 mM KCl-based buffer containing 0.5 μM rotenone, 0.2 mg/mL antimycin A, 2 μM calcium ionophore A23187, 0.5 mM PPi, and 2 mM nitrilotriacetic acid. Swelling and calcium retention capacity (CRC) experiments using respiring mitochondria were performed at 37°C in a 125 mM KCl-based buffer, including 2 mM Pi (K), 200 μM ATP, 50 μM ADP, 1 mM MgCl2, 5 mM malate, and 5 mM glutamate. The extent of swelling was calculated as the calcium-induced decrease in light scattering compared to that by the ionophore alamethicin (10 μg/mL; Hansson et al., 2004a). Due to some variability between mitochondrial preparations, the Ca2+ concentrations administered to the mitochondrial suspensions ranged from 80 to 150 μM, to reach a comparable inter-experimental control response. The same concentration was used for all experiments of a particular preparation. Mitochondrial calcium uptake and release were monitored by the excitation ratio (ex. 340/380 nm, em. 509 nm) of the extramitochondrial calcium-sensitive fluorescent probe Fura 6F (250 nM). The brain mitochondrial suspensions were infused with 77–200 nmol CaCl2/(mg × min). In one set of experiments (Fig. 1B), the infusion speed was initially at the lowest range, but then doubled following infusion of 2.5 μmol/mg CaCl2 in order to reduce experimental time. Liver mitochondria were infused with 50 nmol CaCl2/(mg × min). CRC was calculated as the amount of infused calcium from the start of mitochondrial calcium uptake, until start of maximal calcium release. Rhodamine 123 (100 nM) was used to assess mitochondrial membrane potential, with excitation and emission set to 490 nm and 528 nm, respectively.
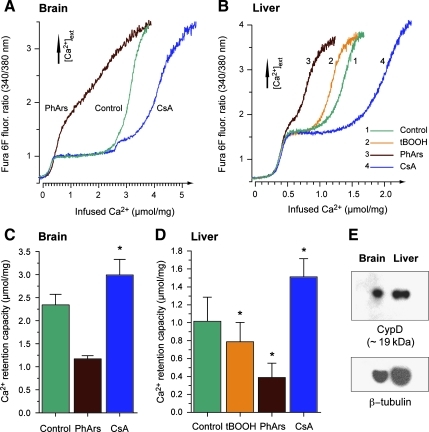
FIG. 1.
Modulation of calcium retention capacity in human brain and liver mitochondria by cyclophilin D (CypD) inhibition and oxidants. (A and B) Representative traces of human brain and liver mitochondrial calcium handling during continuous calcium infusion. Changes in fura 6F fluorescence intensity correspond to the level of extramitochondrial [Ca2+]. Experiments were performed with or without presence of the CypD-inhibitor cyclosporin A (CsA, 1 μM), the vicinal thiol reagent phenylarsine oxide (PhArs, 1 μM), and tert-butyl hydroperoxide (tBOOH, 500 μM, tested in liver mitochondria only). (C and D) Calculated mitochondrial calcium retention capacity. Values are means ± standard error of the mean (SEM; * in C indicates p < 0.05 by paired t-test, n = 4 for control and CsA; PhArs was only tested twice in brain mitochondria and no statistical evaluation was performed; * in D indicates p < 0.05 by repeated-measures ANOVA with Dunnett's post-hoc test, n = 3). (E) Western blots of CypD and β-tubulin in isolated human brain and liver mitochondria (12 μg/lane and 22 μg/lane were used for brain and liver mitochondria, respectively).
Mitochondrial respiration
Respiratory activities of mitochondrial preparations were measured by determining oxygen consumption in airtight chambers at 30°C, using Clark-type oxygen electrodes (Hansatech, Norfolk, U.K.). First, 100 μg brain or 200 μg liver mitochondria were suspended in 400 μL respiration medium consisting of 5 mM malate, 5 mM glutamate, 110 mM sucrose, 60 mM K-lactobionate, 0.5 mM EGTA, 1 g/L BSA, 3 mM MgCl2, 20 mM taurine, 10 mM Pi (K), and 20 mM K-HEPES (pH 7.1; Kuznetsov et al., 2004). Respiratory control ratios (RCR) were calculated as the ratio of oxygen consumption during active phosphorylation in the presence of ADP (state 3) to the resting rate after ADP was consumed (state 4), or after addition of 1 μg/mL oligomycin (state 4oligo).
Electron microscopy
Mitochondrial samples were immersed in 2.5% glutaraldehyde in phosphate buffer (pH 7.4) for 2 h, washed in phosphate buffer for 20 min, immersed for 2 h in 1% osmium tetroxide in phosphate buffer, and then dehydrated with graded alcohol and embedded with embedding medium. Sections 50 nm in size were prepared in Reichert-Jung Ultracut-E and stained with 4% uranylacetate, followed by 0.5% lead citrate. Electron micrographs were obtained using a Hitachi H-7000 electron microscope (Hitachi Ltd., Tokyo, Japan).
Cytochrome c release
Liver mitochondrial samples were prepared similarly to the light scattering experiments, and were exposed to 200 μM Ca2+ with or without mPT inhibitors. An ELISA kit for detection of human cytochrome c (Cyt c; Quantikine®; R&D Systems, Inc., Minneapolis, MN) was employed to measure Cyt c release as described previously (Hansson et al., 2008).
Immunoblotting
Samples of isolated mitochondria were boiled with 2 × SDS sample buffer for 10 min. Total protein (12–24 μg/sample) was separated on 4–12% NuPAGE gels (Invitrogen, Carlsbad, CA), transferred to PVDF membranes, and blocked overnight with 5% skim milk in phosphate-buffered saline. A laboratory-generated (F. Shibazaki), and a commercial (PA1-028; Pierce Thermo Fischer Scientific, Rochester, NY), primary rabbit polyclonal antibody against human CypD were used. Anti-tubulin antibody (Sigma-Aldrich, St. Louis, MO), and anti-ANT mouse monoclonal antibody (Calbiochem, San Diego, CA) were used for internal control. The primary antibodies were incubated at room temperature for 1 h. Anti-horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse IgG secondary antibodies (Pierce Thermo Fischer Scientific) were incubated at room temperature for 1 h, and then washed six times with phosphate-buffered saline containing 0.1% Tween 20, and detected on Western blots by the SuperSignal West Dura chemiluminescence detection system (Pierce Thermo Fischer Scientific).
Statistical analysis
All liver mitochondrial experiments were replicated in at least 3–4 separate mitochondrial preparations. Data are presented as means ± standard error of the mean (SEM), and were generally evaluated with analysis of variance (ANOVA), followed by Dunnett's post-hoc test. Paired comparisons were performed for CRC experiments, as all evaluations of treatment effects were performed using mitochondria from the same individuals. The effect of CypD inhibition on brain mitochondrial CRC was evaluated in four, and reversible swelling in three, separate preparations. The level of statistical significance was set at 5%. Other swelling experiments, respiration, and CRC experiments for phenylarsine oxide were only replicated in two separate brain mitochondrial preparations due to limited size of the samples, and no statistical analyses were performed.
Results
Mitochondrial permeability transition in human brain and liver mitochondria is modulated by cyclophilin D and oxidative stress
The isolation procedure resulted in a yield of 0.13–0.97 mg brain mitochondria and 2.4–21 mg liver mitochondria from the different tissue samples (0.13–0.95 g brain tissue and 0.4–3.9 g liver tissue). Due to the restricted availability and variation in the amount of utilizable brain tissue obtained, we were unable to do full sets of experiments on the brain mitochondrial preparations. To investigate the presence of mPT in adult human mitochondria, suspensions of brain and liver mitochondria were exposed to continuous infusions of calcium, and CRC was determined. CRC is a quantitative mPT assay that measures the amount of calcium mitochondria can retain before induction of mPT causes release of the sequestered calcium (Chalmers and Nicholls, 2003; Hansson et al., 2010). In a physiological medium containing adenine nucleotides and Pi, the CypD inhibitor CsA significantly increased CRC in both brain (2.34 ± 0.23 and 2.99 ± 0.34 μmol Ca2+/mg mitochondria, for control and 1 μM CsA, respectively), and liver mitochondria (1.02 ± 0.27 and 1.51 ± 0.20 μmol Ca2+/mg mitochondria, for control and 1 μM CsA, respectively; Fig. 1A–D). Immunoblots confirmed the presence of CypD in the isolated mitochondrial preparations (Fig. 1E). Oxidative stress is considered to sensitize mitochondria to mPT activation through oxidation of critical thiol groups on the mPT pore components (Halestrap et al., 1997; Petronilli et al., 1994). The vicinal thiol reagent phenylarsine oxide (PhArs; 1 μM) demonstrated a clear tendency toward reduced CRC in brain mitochondria (Fig. 1C, n = 2), and a significant reduction of CRC in liver mitochondria (0.39 ± 0.16 μmol Ca2+/mg; Fig. 1D, n = 3). The oxidant tert-butyl hydroperoxide (tBOOH; 500 μM) also significantly reduced CRC in liver mitochondria (0.79 ± 0.22 μmol Ca2+/mg; Fig. 1D).
Loss of respiratory coupling following calcium-induced permeability transition
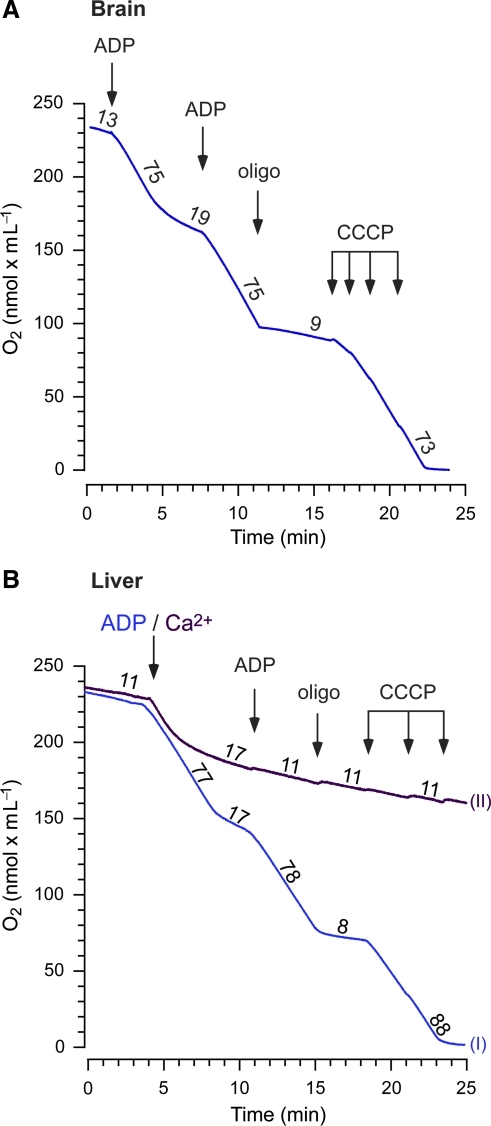
In order to assess the functional integrity of isolated mitochondrial preparations, respiration experiments were performed. Both brain and liver mitochondria demonstrated well-coupled respiration (Fig. 2). Mean respiratory control ratios (RCR) of liver mitochondria were 5.28 ± 0.59 and 9.92 ± 1.2 for state 3/state 4 (n = 5), and state 3/state 4oligo (n = 4), respectively. Brain mitochondria demonstrated an RCR of 3.9 in one complete experiment (Fig. 2A), and 12.6 in a second experiment with a lower concentration of mitochondria (which tended to overestimate the ratio; data not shown). Following exposure to Ca2+, liver mitochondria demonstrated respiratory inhibition and loss of respiratory coupling, as evidenced by a lack of stimulatory responses upon addition of ADP or the protonophore CCCP (carbonyl cyanide m-chlorophenylhydrazone) (Fig. 2B).
FIG. 2.
Coupled respiration in isolated human brain and liver mitochondria with respiratory inhibition following calcium-induced permeability transition. (A and B) Respiratory control was evaluated by measuring oxygen consumption of mitochondria oxidizing 5 mM malate and glutamate during and after ADP phosphorylation (250 μM ADP). A second addition of ADP (1 mM) was followed by administration of the ATP synthase inhibitor oligomycin (oligo, 1 μg/mL), and titration of the protonophore CCCP, 0.5 μM per addition. In liver mitochondria, the first ADP addition (trace I) was replaced by 1 mM CaCl2 (trace II) to induce mPT. Calcium induced an initial stimulation of respiration, followed by respiratory inhibition and abolished respiratory control. The numbers indicate rates of respiration [nmol O2/(min × mg mitochondria)] from one experiment in brain mitochondria, and means from 4–5 experiments in liver mitochondria (CCCP, carbonyl cyanide m-chlorophenylhydrazone; ADP, adenosine diphosphate; ATP, adenosine triphosphate; CaCl2, calcium chloride; mPT, mitochondrial permeability transition). Color image is available online at www.liebertpub.com/neu.
Inhibition of calcium-induced swelling, membrane potential loss, and Cyt c release by cyclosporin analogs and adenine nucleotides in human liver mitochondria
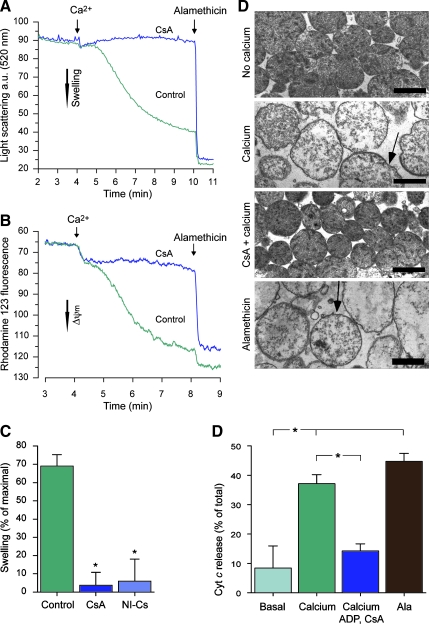
To further evaluate the presence and characteristics of mPT in human mitochondria, mitochondrial morphology, membrane potential, and Cyt c release were examined following calcium exposure. Without cyclosporin analogs present, calcium induced an extensive degree of mitochondrial swelling, 69.0 ± 6.3% compared to that of the non-specific ionophore alamethicin, and mitochondrial membrane potential was essentially lost (Fig. 3A–C). Electron micrographs confirmed a dramatic change in mitochondrial morphology following calcium exposure, including matrix swelling and disruption of the outer mitochondrial membrane (Fig. 3D). The CypD inhibitor CsA, and the non-immunosuppressive cyclosporin analog D-MeAla3EtVal4-cyclosporin (NI-Cs), virtually abolished the calcium-induced swelling (3.67 ± 7.1% and 5.94 ± 12.1%, respectively; Fig. 3A and C), and largely prevented the loss of membrane potential (Fig. 3B). Electron micrographs of mitochondria exposed to calcium in the presence of CsA did not display any overt morphological alterations (Fig. 3D). Calcium exposure also induced an extensive Cyt c release (37.1 ± 3.1% and 8.43 ± 3.8% of total Cyt c content for mitochondria with and without 200 μM Ca2+, respectively; p < 0.05), which was significantly inhibited by ADP and CsA (14.2 ± 1.2% of total Cyt c content; Fig. 3E).
FIG. 3.
Cyclophilin D (CypD)-sensitive swelling, membrane potential dissipation, and cytochrome c release in human liver mitochondria. Respiring human liver mitochondria were exposed to 100 μM Ca2+ with or without the presence of CypD inhibitors, and were monitored by following changes in (A) light scattering, or (B) rhodamine 123 fluorescence. Representative traces of control runs and experiments using the CypD inhibitor cyclosporin A (CsA; 1 μM) are shown. (C) The degree of calcium-induced mitochondrial swelling compared to that induced by the ionophore alamethicin (expressed as a percentage of maximal) was calculated from light-scattering traces for control, CsA, and for the non-immunosuppressive cyclosporin analog D-MeAla3EtVal4-cyclosporin (NI-Cs; 1 μM), using 80–150 μM Ca2+ to induce permeability transition (*p < 0.05 compared to control by analysis of variance [ANOVA] with Dunnett's post-hoc test, n = 3–4). (D) Electron micrographs prepared from light-scattering experiments demonstrating gross morphological swelling and disruption of the outer mitochondrial membrane induced by Ca2+ (arrows). A similar appearance is seen following alamethicin exposure. In presence of CsA, no apparent morphological change was induced by Ca2+ (scale bars = 1 μm). (E) Cytochrome c (Cyt c) release from mitochondria incubated in medium only (control), exposed to 200 μM Ca2+ with or without the mPT inhibitors ADP (200 μM) and CsA (1 μM), and following alamethicin permeabilization (*p < 0.05 between groups by ANOVA with Bonferroni's post-hoc test, n = 4; ADP, adenosine diphosphate; mPT, mitochondrial permeability transition; a.u., arbitrary units). Color image is available online at www.liebertpub.com/neu.
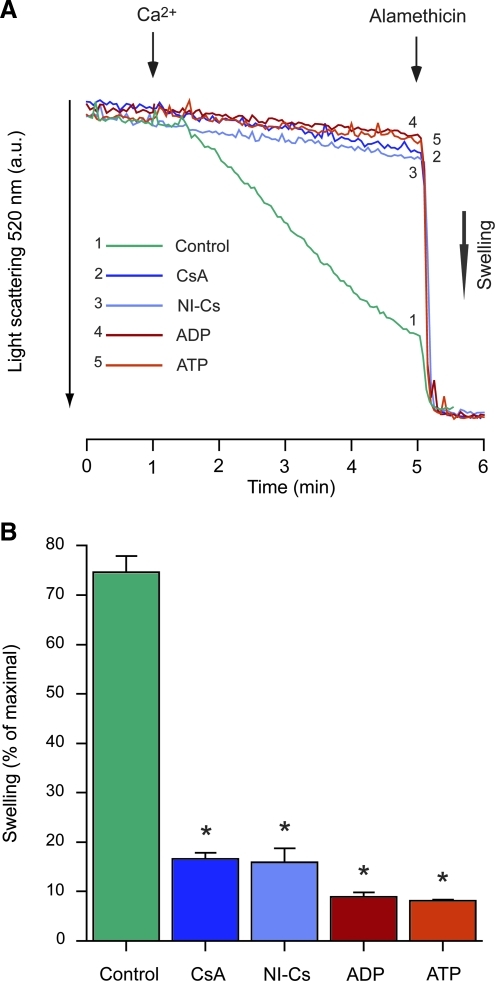
In de-energized mitochondria calcium-induced mPT is independent of respiration-driven electrophoretic Ca2+ uptake, as the anion equilibrates over the mitochondrial inner membrane through a calcium ionophore, and more direct pharmacological interactions with the components of the mPT pore complex can be studied. The CypD inhibitors CsA and NI-Cs (both at 1 μM), as well as the adenine nucleotides ADP and ATP (both at 100 μM), almost completely prevented the swelling induced by 200 μM Ca2+ in de-energized liver mitochondria (Fig. 4A and B).
FIG. 4.
Inhibition of permeability transition by cyclosporin analogs and adenine nucleotides in human liver mitochondria. (A) Representative traces of light-scattering changes of human liver mitochondria exposed to 200 μM Ca2+ under de-energized conditions, in presence of the cyclophilin D (CypD) inhibitors cyclosporin A (CsA; 1 μM), or D-MeAla3EtVal4-cyclosporin (NI-Cs; 1 μM), or the endogenous mPT modulators ADP or ATP (both at 100 μM). (B) Calculations of swelling relative to that induced by alamethicin (percentage of maximal; *p < 0.05 compared to control by analysis of variance with Dunnett's post-hoc test, n = 3; ADP, adenosine diphosphate; ATP, adenosine triphosphate; mPT, mitochondrial permeability transition; a.u., arbitrary units).
Reversible calcium-induced swelling and inhibition of swelling by mPT modulators in human brain mitochondria
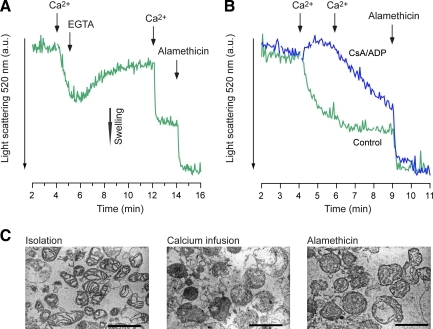
To explore the characteristics of mPT-mediated swelling in human brain mitochondria, the samples were exposed to either transient or long-term calcium exposure. In one set of experiments, 200 μM Ca2+ was administered to mitochondria, EGTA was added after 1 min to chelate the calcium, and the initial light-scattering decrease by calcium was significantly reversed, from 33.9 ± 5.2% to 15.9 ± 4.1% (of alamethicin-induced swelling, n = 3; Fig. 5A). The mitochondria also underwent a pronounced swelling response following a second calcium addition. In another set of experiments human brain mitochondria were exposed to calcium with or without presence of 1 μM CsA and 100 μM ADP. The mPT modulators delayed and partially prevented the swelling response following an addition of a total of 200 μM Ca2+ (n = 2, no statistical analysis was performed; Fig. 5B).
FIG. 5.
Reversible calcium-induced mPT and inhibition of swelling by mPT inhibitors in human brain mitochondria. (A) Representative traces (n = 3) of reversible swelling in human brain mitochondria induced by 200 μM Ca2+, followed by 400 μM of the Ca2+ chelator EGTA. Swelling was induced a second time by Ca2+ (1 mM), and mitochondria were then permeabilized by the non-specific ionophore alamethicin. (B) Representative traces (n = 2) of Ca2+-induced swelling with or without the presence of the endogenous mPT inhibitor ADP (100 μM), and the cyclophilin D (CypD) inhibitor cyclosporin A (CsA; 1 μM). Mitochondria were challenged with two additions of 100 μM Ca2+, followed by exposure to alamethicin. (C) Electron micrographs of brain mitochondria following isolation, calcium infusion, and alamethicin exposure [scale bars = 1 μm; ADP, adenosine diphosphate; mPT, mitochondrial permeability transition; EGTA, ethyleneglycol-bis-(β-aminoethylether)-N,N,N′,N′-tetraacetic acid; a.u., arbitrary units]. Color image is available online at www.liebertpub.com/neu.
Discussion
Mitochondrial dysfunction and activation of mPT are thoroughly implicated in several disorders of the CNS and other organs. Here we demonstrate that both human brain and liver mitochondria exhibit several classical characteristics of the mPT phenomenon following calcium overload. A number of previous conclusions on mPT drawn from animal studies are thus validated in adult human brain and liver mitochondria.
The molecular basis of mPT has been a matter of debate for a couple of decades and has yet to be fully resolved. Seminal studies by Hunter and Haworth established that mPT was induced by Ca2+ and inhibited by ADP, NADH, and Mg2+, and they suggested that mPT may be caused by the regulated opening of a non-specific channel (Haworth and Hunter, 1979; Hunter and Haworth, 1979a). Several lines of evidence pointed toward adenine nucleotide translocator (ANT) as the inner mitochondrial membrane protein mediating mPT (Bauer et al., 1999; Brustovetsky and Klingenberg, 1996; Halestrap and Davidson, 1990; Hunter and Haworth, 1979a). The immunosuppressant CsA was later found to inhibit mPT activation (Crompton et al., 1988), and the effect was attributed to inhibition of the matrix peptidylprolyl cis-trans isomerase CypD, and its interaction with the inner membrane component of mPT (Halestrap and Davidson, 1990). Genetic knockout studies have confirmed an important regulatory role of CypD and ANT, but have questioned that ANT is essential for mPT pore formation (Baines et al., 2005; Basso et al., 2005; Kokoszka et al., 2004; Nakagawa et al., 2005; Schinzel et al., 2005). Recent evidence has also suggested a role of the phosphate carrier in mPT pore formation and regulation (Alcala et al., 2008; Leung et al., 2008), but this awaits the close evaluation that has been performed for ANT and CypD. The present results demonstrate that both human brain and liver mitochondria exhibit several defining characteristics of the mPT phenomenon, such as calcium-induced swelling and calcium-induced calcium release. Human liver mitochondria also demonstrated calcium-induced loss of membrane potential and respiratory coupling, as well as release of Cyt c. These processes were repressed by CypD inhibitors and adenine nucleotides, supporting important roles of CypD and ANT in mPT activation in human mitochondria. Further, oxidative stress is considered to sensitize rodent mitochondria to mPT activation through oxidation of critical thiol groups on the mPT pore components (Halestrap et al., 1997; Petronilli et al., 1994), and both PhArs and tBOOH sensitized human liver mitochondria to mPT.
Corresponding to the intricate regulation of mPT, several classes of drugs have been shown or suggested to inhibit mPT (Kroemer et al., 2007; Zoratti and Szabo, 1995). One difficulty when attributing pharmacological effects to mPT inhibition is that drug effects are often non-specific, both at the mitochondrial level and in vivo (Morota et al., 2009). Another obstacle is that a seemingly beneficial effect on certain mPT characteristics may be caused by inhibition of otherwise vital mitochondrial functions, and the net outcome on mitochondrial integrity may be negative (Mansson et al., 2010). The most common evidence for mPT as a mediator of cell death, and thus its potential as a pharmacological target, derives from studies using CypD inhibitors such as CsA. Even though the target of CsA in mitochondria is specific, in contrast to several other proposed mPT inhibitors (Mansson et al., 2010; Morota et al., 2009), the effect in vivo is not. In order to determine the possible contribution of calcineurin inhibition to the effect of CsA, non-immunosuppressive cyclosporin analogs have been used, but they also inhibit other cyclophilins throughout the cell (Hansson et al., 2004b; Matsumoto et al., 1999; Mbye et al., 2009). A second obstacle when evaluating this class of drugs for neuroprotection is their limited penetration across the blood–brain barrier (BBB; Tsuji et al., 1993), unless measures are taken to facilitate CNS entry. Nevertheless, CsA and its analogs have been among the most convincing and broadly effective group of drugs displaying neuroprotective properties in several diverse models of acute and chronic neurological disease. Pharmacological studies using CypD inhibitors in animal models have in particular implicated mPT in the pathogenesis of focal and global ischemia (Domanska-Janik et al., 2004; Matsumoto et al., 1999; Uchino et al., 1998; Yoshimoto and Siesjö, 1999), hypoglycemic brain damage (Friberg et al., 1998), TBI (Buki et al., 1999; Mbye et al., 2009; Sullivan et al., 2000), and ALS (Karlsson et al., 2004; Keep et al., 2001; Kirkinezos et al., 2004).
More specific evidence for a pathogenic role of mPT and CypD in neurological disorders has been obtained through genetic knockout studies. Animals lacking CypD have displayed increased resistance to cerebral ischemia, supporting the pharmacological studies using CypD inhibitors in different cerebral ischemia models (Schinzel et al., 2005). CypD deletion has also been found to be beneficial in animal models of multiple sclerosis and Alzheimer's disease (Du et al., 2008; Forte et al., 2007).
In contrast to the conclusions of pharmacological studies using CypD inhibitors in animal models of neurological disorders, several studies using isolated mitochondria have questioned a prominent role of mPT in the CNS. Brain mitochondria have been argued to be insensitive to mPT and swelling (Berman et al., 2000), or to be relatively resistant to mPT induction (Andreyev and Fiskum, 1999), and CsA has been suggested to be a less potent inhibitor of mPT in brain mitochondria compared to mitochondria from other organs (Brustovetsky and Dubinsky, 2000; Kristal and Dubinsky, 1997). Other studies have provided evidence and argued for a qualitatively and pharmacologically similar mPT phenomenon in rodent brain mitochondria to that in the more commonly studied heart and liver mitochondria (Chalmers and Nicholls, 2003; Hansson et al., 2004a). Further, it has been suggested that CypD is downregulated in the mature rodent brain (Eliseev et al., 2007). If CypD is downregulated in the adult human brain, there would be no rationale for using CypD inhibitors in patients with neurological disorders. Here we confirm the presence of CypD in human adult brain and liver mitochondria. Further, the CypD inhibitor CsA was found to have a significant effect on mitochondrial CRC, a quantitative assay for mPT, in adult human brain as well as liver mitochondria.
Induction of mPT is transient when inducing factors are removed in vitro (Hunter and Haworth, 1979b). Reversible mPT-mediated swelling has previously been demonstrated in rodent brain mitochondria (Hansson et al., 2004a), and reversible mPT-dependent remodeling of mitochondria has also been described in cultured hippocampal neurons in models of excitotoxicity (Shalbuyeva et al., 2006). Using in vivo imaging with two-photon microscopy, loss of mitochondrial membrane potential has been demonstrated to occur within 1–3 min of global cerebral ischemia. The mitochondrial dysfunction was recovered rapidly upon reperfusion, and was blocked by CsA, indicating that mPT activation is an early reversible event that could trigger delayed cell death (Liu and Murphy, 2009). Previous studies support this conclusion, as mitochondria have been demonstrated to accumulate calcium shortly after ischemia (Zaidan and Sims, 1994), and to undergo transient swelling following ischemia (Petito and Pulsinelli, 1984). Moreover, early CsA administration following reperfusion prevents early Cyt c release, and dramatically reduces delayed neuronal cell death in animals subjected to global ischemia (Domanska-Janik et al., 2004; Uchino et al., 1998). As demonstrated here, mPT activation and swelling are reversible events in human brain mitochondria, supporting the conclusion drawn from animal studies of reversible mPT in the pathogenesis of delayed neuronal death in cerebral ischemia.
As stated above, there is extensive documentation of a neuroprotective effect of CsA in different animal models of TBI. The shear forces will cause an immediate, but also a delayed, cellular injury and disruption of the BBB (Buki and Povlishock, 2006). In contrast to other neurological indications, mPT inhibition can be achieved in brain parenchyma via systemic CsA administration, due to the disruption of the BBB. Promising animal data have influenced two independent groups to initiate NIH-sponsored human clinical trials of CsA administration to patients with severe TBI in the United States. The initial studies in patients with TBI show that CsA is well tolerated, enters the CNS, and demonstrates a dose-related improvement in favorable outcome (Hatton et al., 2008; Mazzeo et al., 2008, 2006). Although mPT inhibition may not be the only target for CsA, these clinical trials in TBI, as well as the promising effect of CsA against myocardial reperfusion injury (Mewton et al., 2010; Piot et al., 2008), are the first human studies testing the hypothesis of mPT-mediated injury in human disease.
The major challenge of the present study was to obtain viable brain tissue and viable mitochondria for functional analyses. Therefore, it was not feasible to address all aspects of mPT in brain mitochondria. Further, brain tissue samples were derived from patients with neurological conditions requiring neurosurgery (Table 1), and it is unknown how these acute conditions may affect the evaluation of mPT and expression of CypD. In summary, we have provided evidence that human brain and liver mitochondria possess a CypD-sensitive permeability transition. Taking the limitations mentioned above into account, the findings presented here support the rationale of CypD inhibition as a pharmacological target in patients with acute neurological damage and chronic neurodegenerative disease.
Acknowledgments
This study was supported by the Swedish Research Council (reference no. 2008-2634), by the Japanese Ministry of Health, Labour and Welfare grant no. 18591724, and the Foundation of the Swedish National Board of Health and Welfare. The authors are grateful to Hitoshi Miura, Takashi Ogata, Hiroshi Nishioka, Tetsuo Ishizaki, Tomohiro Nomura, Mamoru Murakami, Shingo Ohno, Hiroyuki Jinbo, and Hitoshi Izawa for support in obtaining tissue samples. D-MeAla3EtVal4-cyclosporin (Debio-025) was kindly provided by Debiopharm S.A.
Author Disclosure Statement
E.E. is co-founder and officer, and M.J.H. is a stockholder of Maas Biolab, LLC, and NeuroVive Pharmaceutical AB (publ), which hold intellectual property rights and develop the use of cyclosporins as cyclophilin D inhibitors for neurological treatment. The other authors have nothing to disclose.
References
- Alcala S. Klee M. Fernandez J. Fleischer A. Pimentel-Muinos F.X. A high-throughput screening for mammalian cell death effectors identifies the mitochondrial phosphate carrier as a regulator of cytochrome c release. Oncogene. 2008;27:44–54. doi: 10.1038/sj.onc.1210600. [DOI] [PubMed] [Google Scholar]
- Andreyev A. Fiskum G. Calcium induced release of mitochondrial cytochrome c by different mechanisms selective for brain versus liver. Cell Death Differ. 1999;6:825–832. doi: 10.1038/sj.cdd.4400565. [DOI] [PubMed] [Google Scholar]
- Baines C.P. Kaiser R.A. Purcell N.H. Blair N.S. Osinska H. Hambleton M.A. Brunskill E.W. Sayen M.R. Gottlieb R.A. Dorn G.W. Robbins J. Molkentin J.D. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Baines C.P. The cardiac mitochondrion: nexus of stress. Annu. Rev. Physiol. 2010;72:61–80. doi: 10.1146/annurev-physiol-021909-135929. [DOI] [PubMed] [Google Scholar]
- Basso E. Fante L. Fowlkes J. Petronilli V. Forte M.A. Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J. Biol. Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- Bauer M.K. Schubert A. Rocks O. Grimm S. Adenine nucleotide translocase-1, a component of the permeability transition pore, can dominantly induce apoptosis. J. Cell Biol. 1999;147:1493–1502. doi: 10.1083/jcb.147.7.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman S.B. Watkins S.C. Hastings T.G. Quantitative biochemical and ultrastructural comparison of mitochondrial permeability transition in isolated brain and liver mitochondria: evidence for reduced sensitivity of brain mitochondria. Exp. Neurol. 2000;164:415–425. doi: 10.1006/exnr.2000.7438. [DOI] [PubMed] [Google Scholar]
- Bernardi P. Krauskopf A. Basso E. Petronilli V. Blalchy-Dyson E. Di Lisa F. Forte M.A. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- Brustovetsky N. Dubinsky J.M. Limitations of cyclosporin A inhibition of the permeability transition in CNS mitochondria. J. Neurosci. 2000;20:8229–8237. doi: 10.1523/JNEUROSCI.20-22-08229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustovetsky N. Klingenberg M. Mitochondrial ADP/ATP carrier can be reversibly converted into a large channel by Ca2+ Biochemistry. 1996;35:8483–8488. doi: 10.1021/bi960833v. [DOI] [PubMed] [Google Scholar]
- Buki A. Povlishock J.T. All roads lead to disconnection?—Traumatic axonal injury revisited. Acta Neurochir. (Wien.) 2006;148:181–193. doi: 10.1007/s00701-005-0674-4. ; discussion 193–184. [DOI] [PubMed] [Google Scholar]
- Buki A. Okonkwo D.O. Povlishock J.T. Postinjury cyclosporin A administration limits axonal damage and disconnection in traumatic brain injury. J. Neurotrauma. 1999;16:511–521. doi: 10.1089/neu.1999.16.511. [DOI] [PubMed] [Google Scholar]
- Chalmers S. Nicholls D.G. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J. Biol. Chem. 2003;278:9062–19070. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- Cook A.M. Whitlow J. Hatton J. Young B. Cyclosporine A for neuroprotection: establishing dosing guidelines for safe and effective use. Expert Opin. Drug Saf. 2009;8:411–419. doi: 10.1517/14740330903066742. [DOI] [PubMed] [Google Scholar]
- Crompton M. Ellinger H. Costi A. Inhibition by cyclosporin A of a Ca2+ -dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem. J. 1988;255:357–360. [PMC free article] [PubMed] [Google Scholar]
- Domanska-Janik K. Buzanska L. Dluzniewska J. Kozlowska H. Sarnowska A. Zablocka B. Neuroprotection by cyclosporin A following transient brain ischemia correlates with the inhibition of the early efflux of cytochrome C to cytoplasm. Brain Res. Mol. Brain Res. 2004;121:50–59. doi: 10.1016/j.molbrainres.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Du H. Guo L. Fang F. Chen D. Sosunov A.A. McKhann G.M. Yan Y. Wang C. Zhang H. Molkentin J.D. Gunn-Moore F.J. Vonsattel J.P. Arancio O. Chen J.X. Yan S.D. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat. Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliseev R.A. Filippov G. Velos J. VanWinkle B. Goldman A. Rosier R.N. Gunter T.E. Role of cyclophilin D in the resistance of brain mitochondria to the permeability transition. Neurobiol. Aging. 2007;28:1532–1542. doi: 10.1016/j.neurobiolaging.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Empey P.E. McNamara P.J. Young B. Rosbolt M.B. Hatton J. Cyclosporin A disposition following acute traumatic brain injury. J. Neurotrauma. 2006;23:109–116. doi: 10.1089/neu.2006.23.109. [DOI] [PubMed] [Google Scholar]
- Forte M. Gold B.G. Marracci G. Chaudhary P. Basso E. Johnsen D. Yu X. Fowlkes J. Bernardi P. Bourdette D. Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc. Natl. Acad. Sci. USA. 2007;104:7558–7563. doi: 10.1073/pnas.0702228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friberg H. Ferrand-Drake M. Bengtsson F. Halestrap A.P. Wieloch T. Cyclosporin A, but not FK 506, protects mitochondria and neurons against hypoglycemic damage and implicates the mitochondrial permeability transition in cell death. J. Neurosci. 1998;18:5151–5159. doi: 10.1523/JNEUROSCI.18-14-05151.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A.P. Davidson A.M. Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem. J. 1990;268:153–160. doi: 10.1042/bj2680153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A.P. Pasdois P. The role of the mitochondrial permeability transition pore in heart disease. Biochim. Biophys. Acta. 2009;1787:1402–1415. doi: 10.1016/j.bbabio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Halestrap A.P. Woodfield K.Y. Connern C.P. Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. J. Biol. Chem. 1997;272:3346–3354. doi: 10.1074/jbc.272.6.3346. [DOI] [PubMed] [Google Scholar]
- Hansson M.J. Mansson R. Mattiasson G. Ohlsson J. Karlsson J. Keep M.F. Elmer E. Brain-derived respiring mitochondria exhibit homogeneous, complete and cyclosporin-sensitive permeability transition. J. Neurochem. 2004a;89:715–729. doi: 10.1111/j.1471-4159.2004.02400.x. [DOI] [PubMed] [Google Scholar]
- Hansson M.J. Mansson R. Morota S. Uchino H. Kallur T. Sumi T. Ishii N. Shimazu M. Keep M.F. Jegorov A. Elmer E. Calcium-induced generation of reactive oxygen species in brain mitochondria is mediated by permeability transition. Free Radic. Biol. Med. 2008;45:284–294. doi: 10.1016/j.freeradbiomed.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Hansson M.J. Mattiasson G. Mansson R. Karlsson J. Keep M.F. Waldmeier P. Ruegg U.T. Dumont J.M. Besseghir K. Elmer E. The nonimmunosuppressive cyclosporin analogs NIM811 and UNIL025 display nanomolar potencies on permeability transition in brain-derived mitochondria. J. Bioenerg. Biomembr. 2004b;36:407–413. doi: 10.1023/B:JOBB.0000041776.31885.45. [DOI] [PubMed] [Google Scholar]
- Hansson M.J. Morota S. Teilum M. Mattiasson G. Uchino H. Elmer E. Increased potassium conductance of brain mitochondria induces resistance to permeability transition by enhancing matrix volume. J. Biol. Chem. 2010;285:741–750. doi: 10.1074/jbc.M109.017731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton J. Rosbolt B. Empey P. Kryscio R. Young B. Dosing and safety of cyclosporine in patients with severe brain injury. J. Neurosurg. 2008;109:699–707. doi: 10.3171/JNS/2008/109/10/0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth R.A. Hunter D.R. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch. Biochem. Biophys. 1979;195:460–467. doi: 10.1016/0003-9861(79)90372-2. [DOI] [PubMed] [Google Scholar]
- Hunter D.R. Haworth R.A. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch. Biochem. Biophys. 1979a;195:453–459. doi: 10.1016/0003-9861(79)90371-0. [DOI] [PubMed] [Google Scholar]
- Hunter D.R. Haworth R.A. The Ca2+-induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch. Biochem. Biophys. 1979b;195:468–477. doi: 10.1016/0003-9861(79)90373-4. [DOI] [PubMed] [Google Scholar]
- Karlsson J. Fong K.S. Hansson M.J. Elmer E. Csiszar K. Keep M.F. Life span extension and reduced neuronal death after weekly intraventricular cyclosporin injections in the G93A transgenic mouse model of amyotrophic lateral sclerosis. J. Neurosurg. 2004;101:128–137. doi: 10.3171/jns.2004.101.1.0128. [DOI] [PubMed] [Google Scholar]
- Keep M. Elmér E. Fong K.S. Csiszar K. Intrathecal cyclosporin prolongs survival of late-stage ALS mice. Brain Res. 2001;894:327–331. doi: 10.1016/s0006-8993(01)02012-1. [DOI] [PubMed] [Google Scholar]
- Kirkinezos I.G. Hernandez D. Bradley W.G. Moraes C.T. An ALS mouse model with a permeable blood-brain barrier benefits from systemic cyclosporine A treatment. J. Neurochem. 2004;88:821–826. doi: 10.1046/j.1471-4159.2003.02181.x. [DOI] [PubMed] [Google Scholar]
- Kokoszka J.E. Waymire K.G. Levy S.E. Sligh J.E. Cai J. Jones D.P. MacGregor G.R. Wallace D.C. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristal B.S. Dubinsky J.M. Mitochondrial permeability transition in the central nervous system: induction by calcium cycling-dependent and -independent pathways. J. Neurochem. 1997;69:524–538. doi: 10.1046/j.1471-4159.1997.69020524.x. [DOI] [PubMed] [Google Scholar]
- Kroemer G. Galluzzi L. Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- Kuznetsov A.V. Schneeberger S. Seiler R. Brandacher G. Mark W. Steurer W. Saks V. Usson Y. Margreiter R. Gnaiger E. Mitochondrial defects and heterogeneous cytochrome c release after cardiac cold ischemia and reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1633–H1641. doi: 10.1152/ajpheart.00701.2003. [DOI] [PubMed] [Google Scholar]
- Leung A.W. Varanyuwatana P. Halestrap A.P. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J. Biol. Chem. 2008;283:26312–26323. doi: 10.1074/jbc.M805235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.R. Murphy T.H. Reversible cyclosporin A-sensitive mitochondrial depolarization occurs within minutes of stroke onset in mouse somatosensory cortex in vivo: a two-photon imaging study. J. Biol. Chem. 2009;284:36109–36117. doi: 10.1074/jbc.M109.055301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansson R. Morota S. Hansson M.J. Sonoda I. Yasuda Y. Shimazu M. Sugiura A. Yanagi S. Miura H. Uchino H. Elmer E. Minocycline sensitizes rodent and human liver mitochondria to the permeability transition: implications for toxicity in liver transplantation. Hepatology (Baltimore) 2010;51:347–348. doi: 10.1002/hep.23465. ; author reply 349–350. [DOI] [PubMed] [Google Scholar]
- Martin L.J. Gertz B. Pan Y. Price A.C. Molkentin J.D. Chang Q. The mitochondrial permeability transition pore in motor neurons: involvement in the pathobiology of ALS mice. Exp. Neurol. 2009;218:333–346. doi: 10.1016/j.expneurol.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S. Friberg H. Ferrand-Drake M. Wieloch T. Blockade of the mitochondrial permeability transition pore diminishes infarct size in the rat after transient middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 1999;19:736–741. doi: 10.1097/00004647-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Mazzeo A.T. Alves O.L. Gilman C.B. Hayes R.L. Tolias C. Niki Kunene K. Ross Bullock M. Brain metabolic and hemodynamic effects of cyclosporin A after human severe traumatic brain injury: a microdialysis study. Acta Neurochir. (Wien.) 2008;150:1019–1031. doi: 10.1007/s00701-008-0021-7. ; discussion 1031. [DOI] [PubMed] [Google Scholar]
- Mazzeo A.T. Kunene N.K. Gilman C.B. Hamm R.J. Hafez N. Bullock M.R. Severe human traumatic brain injury, but not cyclosporin a treatment, depresses activated T lymphocytes early after injury. J. Neurotrauma. 2006;23:962–975. doi: 10.1089/neu.2006.23.962. [DOI] [PubMed] [Google Scholar]
- Mbye L.H. Singh I.N. Carrico K.M. Saatman K.E. Hall E.D. Comparative neuroprotective effects of cyclosporin A and NIM811, a nonimmunosuppressive cyclosporin A analog, following traumatic brain injury. J. Cereb. Blood Flow Metab. 2009;29:87–97. doi: 10.1038/jcbfm.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewton N. Croisille P. Gahide G. Rioufol G. Bonnefoy E. Sanchez I. Cung T.T. Sportouch C. Angoulvant D. Finet G. Andre-Fouet X. Derumeaux G. Piot C. Vernhet H. Revel D. Ovize M. Effect of cyclosporine on left ventricular remodeling after reperfused myocardial infarction. J. Am. Coll. Cardiol. 2010;55:1200–1205. doi: 10.1016/j.jacc.2009.10.052. [DOI] [PubMed] [Google Scholar]
- Millay D.P. Sargent M.A. Osinska H. Baines C.P. Barton E.R. Vuagniaux G. Sweeney H.L. Robbins J. Molkentin J.D. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat. Med. 2008;14:442–447. doi: 10.1038/nm1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morota S. Mansson R. Hansson M.J. Kasuya K. Shimazu M. Hasegawa E. Yanagi S. Omi A. Uchino H. Elmer E. Evaluation of putative inhibitors of mitochondrial permeability transition for brain disorders—Specificity vs. toxicity. Exp. Neurol. 2009;218:353–362. doi: 10.1016/j.expneurol.2009.03.036. [DOI] [PubMed] [Google Scholar]
- Nakagawa T. Shimizu S. Watanabe T. Yamaguchi O. Otsu K. Yamagata H. Inohara H. Kubo T. Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Nicholls D.G. Mitochondrial calcium function and dysfunction in the central nervous system. Biochim. Biophys. Acta. 2009;1787:1416–1424. doi: 10.1016/j.bbabio.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito C.K. Pulsinelli W.A. Delayed neuronal recovery and neuronal death in rat hippocampus following severe cerebral ischemia: possible relationship to abnormalities in neuronal processes. J. Cereb. Blood Flow Metab. 1984;4:194–205. doi: 10.1038/jcbfm.1984.28. [DOI] [PubMed] [Google Scholar]
- Petronilli V. Costantini P. Scorrano L. Colonna R. Passamonti S. Bernardi P. The voltage sensor of the mitochondrial permeability transition pore is tuned by the oxidation-reduction state of vicinal thiols. Increase of the gating potential by oxidants and its reversal by reducing agents. J. Biol. Chem. 1994;269:16638–16642. [PubMed] [Google Scholar]
- Piot C. Croisille P. Staat P. Thibault H. Rioufol G. Mewton N. Elbelghiti R. Cung T.T. Bonnefoy E. Angoulvant D. Macia C. Raczka F. Sportouch C. Gahide G. Finet G. Andre-Fouet X. Revel D. Kirkorian G. Monassier J.P. Derumeaux G. Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N. Engl. J. Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- Russmann S. Kullak-Ublick G.A. Grattagliano I. Current concepts of mechanisms in drug-induced hepatotoxicity. Curr. Med. Chem. 2009;16:3041–3053. doi: 10.2174/092986709788803097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinzel A.C. Takeuchi O. Huang Z. Fisher J.K. Zhou Z. Rubens J. Hetz C. Danial N.N. Moskowitz M.A. Korsmeyer S.J. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc. Natl. Acad. Sci. USA. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalbuyeva N. Brustovetsky T. Bolshakov A. Brustovetsky N. Calcium-dependent spontaneously reversible remodeling of brain mitochondria. J. Biol. Chem. 2006;281:37547–37558. doi: 10.1074/jbc.M607263200. [DOI] [PubMed] [Google Scholar]
- Sims N.R. Anderson M.F. Isolation of mitochondria from rat brain using Percoll density gradient centrifugation. Nature Protocols. 2008;3:1228–1239. doi: 10.1038/nprot.2008.105. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Rabchevsky A.G. Hicks R.R. Gibson T.R. Fletcher-Turner A. Scheff S.W. Dose-response curve and optimal dosing regimen of cyclosporin A after traumatic brain injury in rats. Neuroscience. 2000;101:289–295. doi: 10.1016/s0306-4522(00)00380-8. [DOI] [PubMed] [Google Scholar]
- Tsuji A. Tamai I. Sakata A. Tenda Y. Terasaki T. Restricted transport of cyclosporin A across the blood-brain barrier by a multidrug transporter, P-glycoprotein. Biochem. Pharmacol. 1993;46:1096–1099. doi: 10.1016/0006-2952(93)90677-o. [DOI] [PubMed] [Google Scholar]
- Uchino H. Elmér E. Uchino K. Li P.A. He Q.P. Smith M.L. Siesjö B.K. Amelioration by cyclosporin A of brain damage in transient forebrain ischemia in the rat. Brain Res. 1998;812:216–226. doi: 10.1016/s0006-8993(98)00902-0. [DOI] [PubMed] [Google Scholar]
- Waldmeier P.C. Zimmermann K. Qian T. Tintelnot-Blomley M. Lemasters J.J. Cyclophilin D as a drug target. Curr. Med. Chem. 2003;10:1485–1506. doi: 10.2174/0929867033457160. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T. Siesjö B.K. Posttreatment with the immunosuppressant cyclosporin A in transient focal ischemia. Brain Res. 1999;839:283–291. doi: 10.1016/s0006-8993(99)01733-3. [DOI] [PubMed] [Google Scholar]
- Zaidan E. Sims N.R. The calcium content of mitochondria from brain subregions following short-term forebrain ischemia and recirculation in the rat. J. Neurochem. 1994;63:1812–1819. doi: 10.1046/j.1471-4159.1994.63051812.x. [DOI] [PubMed] [Google Scholar]
- Zoratti M. Szabo I. The mitochondrial permeability transition. Biochim. Biophys. Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]