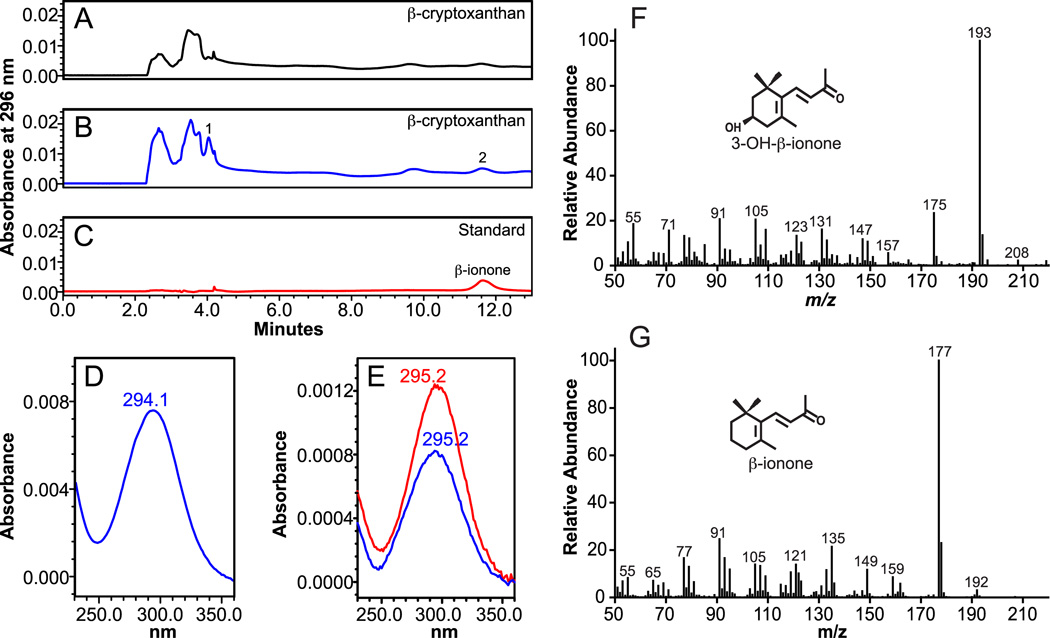
Fig 6.
Identification of volatile cleavage products from β-cryptoxanthin by HPLC and GC-MS analysis. β-Cryptoxanthin (100 µM) was incubated with homogenates from either uninfected (A) or ferret CMO2-baculovirus infected (B) insect cell lysates for 30 min. at 37°C as described in “Experimental Procedures”. The cleavage products were extracted from the incubation mixture and separated by reverse-phase HPLC using a C18 column. Unknown reaction products 1 and 2 were detected at 296 nm only in the incubation mixtures with ferret CMO2-baculovirus infected cells (B) but not in that of the uninfected cells (A). Spectral analysis of reaction products 1 (D) and 2 (E), which matched in retention time and absorption spectra of the β-ionone standard, indicated the presence of ionone compounds. GC-MS analysis of reactions product 1 from β-cryptoxanthin cleavage indicated the presence of 3-OH-β-ionone (F) and analysis of reaction product 2 indicated the presence of β-ionone (G). The EI mass spectra were matched to spectra found in literature [38, 39].