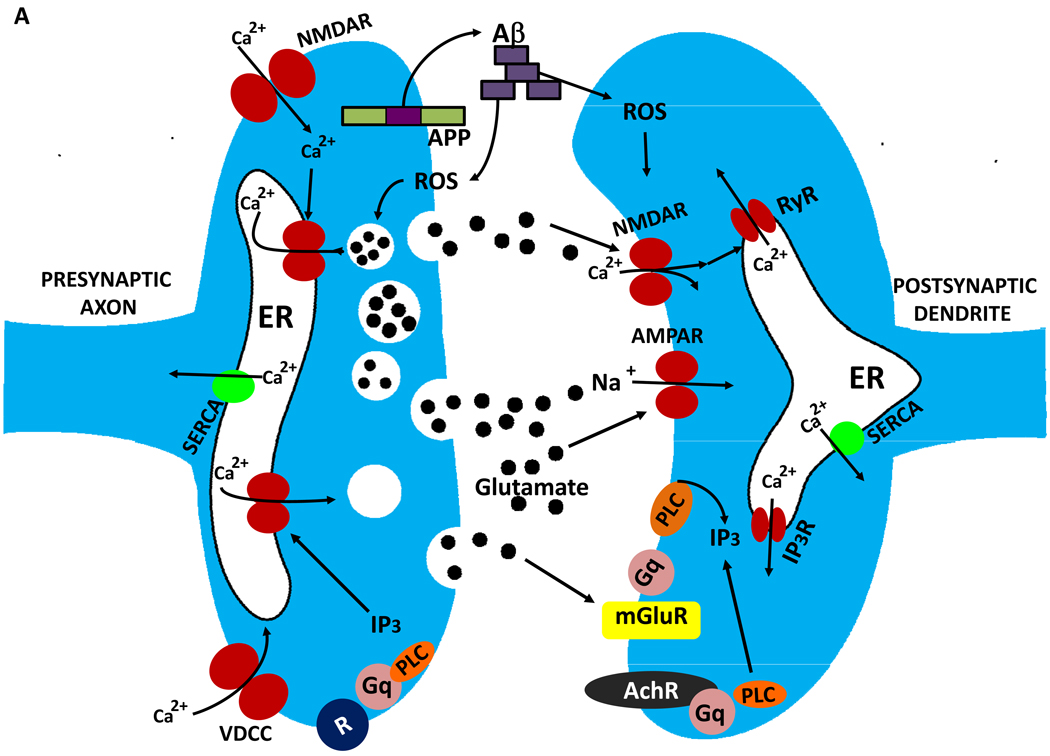
Figure 4.
Mechanisms of synaptic dysfunction in aging and Alzheimer’s disease. The β-amyloid precursor protein is axonally transported and so is present in high amounts in presynaptic terminals. In properly functioning synapses the APP is proteolytically cleaved in the middle of the Aβ sequence by the α-secretase, thereby preventing the production of Aβ. During normal aging, and more so in AD, APP is cleaved at the N- and C-termini of Aβ by β-secretase and γ-secretase, respectively, resulting in the production and self-aggregation of Aβ. Aggregation of Aβ on the membrane generates ROS resulting in membrane lipid peroxidation, which then impairs the function of membrane ion-motive ATPases thereby promoting membrane depolarization and Ca2+ influx through NMDA receptor channels and voltage-dependent Ca2+ channels. Sustained elevation of cytoplasmic Ca2+ levels promotes depletion of presynaptic glutamate stores resulting in impaired synaptic transmission and damage to axons and dendrites. In addition, perturbed mitochondrial function caused by aging, oxidative stress and Aβ results in energy depletion in neurons which exacerbates synaptic dysfunction and degeneration of neurons. Further contributing to the demise of neurons in AD is dysregulation of endoplasmic reticulum (ER) function that results in depletion of ER Ca2+ stores and accumulation of misfolded proteins.