Abstract
Electromyography (EMG) of the shoulder girdle is commonly performed; however, EMG spectral properties of shoulder muscles have not been clearly defined. The purpose of this study was to determine the maximum power frequency, Nyquist rate, and minimum sampling rate for indwelling and surface EMG of the normal shoulder girdle musculature. EMG signals were recorded using indwelling electrodes for the rotator cuff muscles and surface electrodes for ten additional shoulder muscles in ten healthy volunteers. A fast Fourier transform was performed on the raw EMG signal collected during maximal isometric contractions to derive the power spectral density. The 95% power frequency was calculated during the ramp and plateau subphase of each contraction. Data were analyzed with analysis of variance (ANOVA) and paired t tests. Indwelling EMG signals had more than twice the frequency content of surface EMG signals (p < .001). Mean 95% power frequencies ranged from 495 to 560 Hz for indwelling electrodes and from 152 to 260 Hz for surface electrodes. Significant differences in the mean 95% power frequencies existed among muscles monitored with surface electrodes (p = .002), but not among muscles monitored with indwelling electrodes (p = .961). No significant differences in the 95% power frequencies existed among contraction subphases for any of the muscle–electrode combinations. Maximum Nyquist rate was 893 Hz for surface electrodes and 1,764 Hz for indwelling electrodes. Our results suggest that when recording EMG of shoulder muscles, the minimum sampling frequency is 1,340 Hz for surface electrodes and 2,650 Hz for indwelling electrodes. The minimum sampling recommendations are higher than the 1,000 Hz reported in many studies involving EMG of the shoulder.
Keywords: shoulder, rotator cuff, electromyography, isometric contraction
Introduction
Kinesiologic electromyography (EMG) is one of the tools available for researchers and clinicians to evaluate muscle function and activation patterns during functional tasks [29, 35]. Selection of the type of electrode used during these measurements is based upon several factors, including the location of the muscle of interest, the need for specificity, and the requirement of minimization of cross-talk between adjacent muscles. Deep muscles require the use of finewire electrodes, while superficial muscle activity can be detected with surface electrodes [4, 14, 28]. At the shoulder, the subscapularis is inaccessible for recording with surface electrodes, while the anterior deltoid is accessible. In addition, the use of indwelling electrodes allows one to distinguish between the upper and lower portions of the subscapularis muscle [7]. However, as this is an invasive procedure with associated insertional discomfort, the number of indwelling electrodes is often kept to a minimum. Thus, when multiple muscles are studied, some will have EMG recorded with surface electrodes and some with indwelling electrodes [6, 7, 19].
An ad hoc committee of the International Society of Electrophysiological Kinesiology (ISEK) set the original standards for capturing and reporting kinesiologic EMG results. They described the signal frequency range for indwelling (finewire) and surface EMG [38]. Specifically, signal frequencies for indwelling EMG ranged from 0.1 to 10,000 Hz, while surface EMG ranged from 1 to 3,000 Hz. Standards for surface EMG recordings were published by the European Recommendations for Surface Electromyography for the SENIAM project [1]. These recommendations suggest a sampling rate of greater than 1,000 Hz to capture a maximum signal frequency of 500 Hz.
In practice, due to hardware and computing limitations, muscle activity is often measured at the lower end of this range using sampling frequencies from 1,000 to 1,200 Hz and low-pass filtered at 350 to 500 Hz [2, 6]. A disadvantage of undersampling a signal is that any higher frequency signals are discarded. Thus, the recorded signal is distorted as the higher frequencies are aliased as lower frequencies. For signal processing, aliasing refers to the effect that causes the high frequency component of continuous signals, in this case, the EMG signal, to become indistinguishable (or aliased) from the lower frequency component of the signals. This can be prevented by sampling at the Nyquist rate (i.e., a rate that is twice the maximum frequency of interest) [27, 31].
The effect of sample rate selection on surface EMG timing and amplitude is highlighted by a study conducted by Ives and Wigglesworth [16]. The authors collected EMG of the triceps brachii using surface electrodes, a sampling rate of 6 kHz, and an amplifier bandwidth of 20 Hz to 2 kHz. Signals were then resampled at 3, 1, 500, and 250 Hz to determine, post hoc, the effect of lower sampling rates. They determined that the EMG spectrum of this muscle was contained within 500 Hz, and thus, the Nyquist rate was 1 kHz. Undersampling at 500 and 250 Hz had significant effects on the accuracy of the onset latency and burst duration measures, as well as on the amplitude of the signal. They documented an 11.4% decrease in EMG amplitude when the signal was resampled at 250 Hz. When the signal was resampled at 1 kHz, the amplitude decreased by only 2.4%. As amplitude is a common outcome in kinesiologic EMG studies, these findings suggest that investigators should choose an appropriate sampling rate that minimizes aliasing of the signal
EMG spectral properties, or the distribution of frequencies contained within the signal, have been studied in a variety of muscles [17, 22, 23, 31, 32, 36]. Tesch et al. [36] found the quadriceps to have a maximum frequency content of 500 Hz when using surface electrodes. Kumar et al. [22, 23] examined cervical muscles and found the splenius capitis and sternocleidomastoid to have 400- and 200-Hz maximum frequencies, respectively. Schweitzer et al. [32] studied the inspiratory diaphragmatic EMG via esophageal electrodes with a bandwidth of 25 to 250 Hz. However, there is a lack of available literature describing EMG spectral properties of the muscles of the shoulder complex. The EMG spectral characteristics of the middle deltoid [31] and the biceps brachii [12] recorded with surface electrodes were reported to be less than 200 Hz, but these characteristics have not been reported for other shoulder muscles.
While other investigators have published results that detail the median frequency of EMG for shoulder muscles, we found no published studies evaluating the EMG power spectrum of the frequency content of the rotator cuff muscles despite their routine use in research and clinical evaluations of individuals with rotator cuff tears or athletes during sports activities [3, 17, 18, 20, 25, 26]. Therefore, it is important to document the frequency content of the EMG signals of these muscles to ensure that appropriate sampling rates are used. The content may be different for muscles that use indwelling vs. surface electrodes.
To properly select a sampling rate for data collection of kinesiologic EMG for a specific muscle, the highest frequency of interest for the muscle must be known. This can be accomplished with a power spectrum analysis. Once the frequency content of the signal (the spectrum) has been calculated; the frequency that contains 95% of the power of the signal (95% power frequency) can be determined. According to the SENIAM recommendations, the remaining upper 5% of the power spectrum is electrode and equipment noise. The cutoff of 95% power frequency is used in other signal processing algorithms to capture the majority of the signal in the power spectrum and minimize the inclusion of noise in the recorded signal [33, 34].
During maximal voluntary isometric contractions collected as part of routine testing, there is a ramp subphase when muscle activity and force increase from zero until the plateau or peak amplitude is reached. The characteristics of the fibers recruited may be different during these two subphases, and thus, characteristics of the spectrum may be different in these two subphases. During isometric contractions, muscle recruitment occurs according to Henneman size principle [15]. The small muscle fibers with the lowest conduction velocities are recruited first followed by the larger muscle fibers with faster conduction velocities. As the frequency of the EMG is influenced by the conduction velocity of the muscle fibers active during the contraction, the frequency content of the signal can change as force increases and additional fibers are recruited [11, 30]. A spectral shift to higher frequencies occurs as the fast twitch muscles are recruited. The sampling rate should be sufficient to capture data regardless of the subphase, ramp or plateau, during muscle contraction.
Despite recommendations to sample at twice the frequency of interest in a given muscle, real equipment limitations may exist. If equipment limitations only allow data collection at 1,000 Hz, a scenario could occur in which a typical low-pass filter is set at 350 Hz to meet the criteria of a setting <500 Hz (1/2 of the 1,000 Hz sampling rate) [37]. These settings are common practice with previous standards for EMG equipment and software memory limitations. An understanding of the effect of a narrower bandwidth on the recorded signal is needed. The clinical and research question that remains is what amount of signal will be lost if data collection occurs at a low frequency and with a narrow band-pass [5, 10, 20].
To address this problem, we designed a cross sectional study to determine proper sampling rates in select shoulder muscles, as measured with the appropriate electrode type (surface vs. indwelling). The following specific aims were proposed to
determine the 95% power frequency of selected shoulder muscles and electrode combinations;
establish the Nyquist rate for these same muscle and electrode combinations;
test the hypotheses that differences exist in the maximum 95% power frequency between muscles within each electrode type, regardless of subphase and in the mean 95% power frequency between electrode types, regardless of muscle;
test the hypothesis that the 95% power frequency for a given muscle–electrode combination would differ based on the subphase of contraction (ramp vs. plateau); and
determine the percentage of total power spectrum that is retained when shoulder EMG signals are low-pass filtered at 350 Hz.
Materials and methods
The following protocol was approved by our institutional review board, and informed consent was obtained from all enrolled subjects. The ten healthy subjects had no history of musculoskeletal injury or pathology, pain, or surgery in the tested shoulder. No subject currently participated in competitive level sports or activities, which could have caused an asymmetrical training effect. Each subject’s shoulder was evaluated on physical examination by an orthopedic surgeon and on ultrasound by a musculoskeletal radiologist to document an intact rotator cuff. The subjects consisted of four females (age, 33.3 ± 7.2 years; range, 23–39 years) and six males (age, 34.4.3 ± 7.6 years; range, 29–49 years) with the dominant shoulder tested in nine and nondominant shoulder tested in one subject, who requested that the nondominant side be tested to eliminate any risk from indwelling wire insertion in the dominant side.
The EMG activities of 14 muscles were measured with either surface or indwelling electrodes according to a standardized protocol (Table 1) [19, 20]. For each muscle, only those isometric trials where the muscle had either a primary or a secondary role were used for analysis (Table 2). Each EMG signal was measured using the MA300 (Motion Lab Systems, Baton Rouge, LA). The surface electrodes were stainless steel, 12 mm in diameter with an 18-mm interelectrode separation. The premanufactured indwelling electrodes were inserted via 25-gauge hypodermic needles with a 1-cm separation at the insertion site (Cardinal Health, Dublin, OH). Each indwelling electrode was insulated stainless steel, 0.08 mm in diameter with the last 2 mm stripped for an exposed area of 0.51 mm2. Each indwelling electrode was visually inspected prior to insertion. The MA300 system is comprised of surface and wire preamplifier electrode assemblies, a backpack signal conditioning multiplexer circuit that transmits the modulated signal over a single coaxial cable, and a demultiplexer. The preamplifier assemblies incorporate two-pole high- and low-pass filters with a 10-Hz to 3.5-kHz −3-dB response. The backpack contains a tenth-order linear phase root raised cosine low-pass filter that was set at its maximum bandwidth of 2,000 Hz. Each EMG signal was then sampled at 5 kHz using a National Instruments 12 bit A/D converter.
Table 1.
The shoulders muscles tested and the electrode type used for recording EMG signals
| Muscle | Electrode type | |
|---|---|---|
| Surface | Indwelling | |
| Anterior deltoid | × | |
| Middle deltoid | × | |
| Posterior deltoid | × | |
| Pectoralis major | × | |
| Upper trapezius | × | |
| Middle trapezius | × | |
| Lower trapezius | × | |
| Latissimus dorsi | × | |
| Serratus anterior | × | |
| Biceps brachii | × | |
| Supraspinatus | × | |
| Infraspinatus | × | |
| Upper subscapularis | × | |
| Lower subscapularis | × | |
Table 2.
MVIC test positions with primary and secondary muscles used for data analysis
| Primary muscle | Secondary muscles | Isometric contraction |
|---|---|---|
| Anterior deltoid | Biceps brachii | Resisted elevation in scapular plane, shoulder at 0° |
| Pectoralis major | ||
| Middle deltoid | Supraspinatus | Resisted abduction, shoulder at 0° |
| Posterior deltoid | Latissimus dorsi | Resisted extension, shoulder at 0° |
| Pectoralis major | Biceps brachii | Resisted horizontal adduction, shoulder at 90° elevation in scapular plane |
| Latissimus dorsi | ||
| Upper trapezius | – | Resisted shoulder shrug while seated, shoulder at 0° |
| Middle trapezius | – | Scapular retraction, shoulder at 0° |
| Lower trapezius | – | Resisted shoulder flexion, shoulder at maximum elevation |
| Latissimus dorsi | Posterior deltoid | Resisted extension, shoulder at 45° elevation in scapular plane |
| Serratus anterior | – | Shoulder protraction, shoulder at 90° elevation in scapular plane |
| Biceps brachii | – | Resisted elbow flexion, shoulder at 0°, elbow at 90° flexion |
| Supraspinatus | Anterior deltoid | Resisted forward flexion in scapular plane, shoulder at 90° |
| Middle deltoid | ||
| Infraspinatus | Posterior deltoid | Resisted external rotation, shoulder at 0° |
| Upper subscapularis | Anterior deltoid | Resisted internal rotation, shoulder at 0° |
| Pectoralis major | ||
| Latissimus dorsi | ||
| Lower subscapularis | ||
| Lower subscapularis | Anterior deltoid | Resisted internal rotation, shoulder at 90° elevation in scapular plane |
| Pectoralis major | ||
| Latissimus dorsi | ||
| Upper subscapularis |
Muscles where indwelling electrodes were used are italicized
Maximum voluntary isometric contractions (MVICs) were performed for each muscle (Table 2) [5, 19]. Each isometric contraction was divided into two subphases, the ramp and the plateau (Fig. 1), defined as follows. Using Visual3D software (C-Motion, Germantown, MD), the raw EMG signal was first rectified. A linear envelope of the rectified signal was obtained by low-pass filtering at 3.14 Hz. The slope (rate of amplitude change) of the envelope was calculated by taking the first derivative. The raw signal, the linear envelope, and the slope were then exported to MATLAB (Mathworks, Natick, MA) software for further processing.
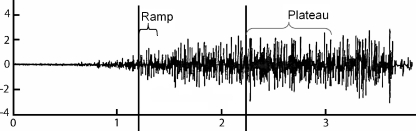
Fig. 1.
A representative surface EMG vs. time curve along with the ramp and plateau phases
The ramp subphase was defined as the 1,024 samples (0.2 seconds) after the first point in the recording where both the slope and the magnitude of the linear envelope exceeded 30% of the maximum slope and magnitude, respectively, over the entire recording. The plateau subphase was defined as the 4,096 samples (0.8 seconds) following the first point in the recording after the ramp subphase where the magnitude of the linear envelope exceeded 70% of its maximum over the entire recording. The 30% and 70% levels provided consistent results when used with the automated algorithm for processing the data. With a percentage <30% of the maximum slope, small changes in slope did not correspond to the beginning of the isometric contraction and gave spurious results. Similarly, at 70% of the maximum of the linear envelope, we captured the portion of the EMG signal during a steady-state contraction.
A fast Fourier transform was then performed on the raw EMG signal data, which were divided into the ramp and plateau subphases, to derive the power spectral density. The 95% power frequency was calculated for each muscle, for both subphases. Also, the maximum 95% power frequency for each muscle was determined as the highest value obtained during a contraction, regardless of subphase. Descriptive statistics, including mean and standard deviations (SDs), were calculated of the 95% power frequency. To determine the upper boundary for data sampling for 95% of the population, the Nyquist rate was calculated for each muscle as follows [27]:
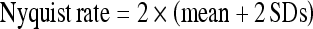 |
Lastly, we calculated what percentage of the power spectrum would be captured in the EMG signal when the bandwidth was set from 10 to 350 Hz instead of the original 10 to 2,500 Hz bandwidth. The power spectra of the signals for all subjects were generated using MATLAB software, and the aggregate power for all frequencies <350 Hz was calculated. The powers still present in this narrower bandwidth were reported as a percentage of the total power contained in all frequencies present in the original 10 to 2,500 Hz bandwidth.
Descriptive statistics were calculated for the 95% power frequencies for both bandwidth settings. An ANOVA was used to determine if a difference existed between the maximum 95% power frequencies among muscles within the same electrode type. The maximum 95% power frequency was averaged within a subject for each electrode type so mean maximum 95% power frequencies for surface and indwelling electrodes were determined. These means for each subject were compared between electrode types with paired t tests. Within each muscle–electrode combination, paired t tests were calculated to determine the effects of subphase. For all statistical tests, a Bonferroni correction was used with an α of .05.
Results
The time and frequency domains of a typical shoulder indwelling EMG signal are shown in Fig. 2. The 2 SDs about the means are plotted in Fig. 3 and listed along with the upper limits (ULs) (mean + 2 SDs) and Nyquist rate in Table 3 for each muscle, for both subphases.
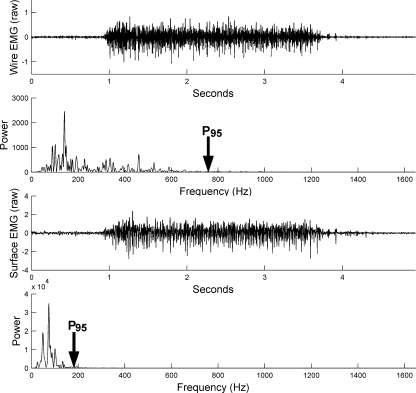
Fig. 2.
The time and frequency domains of a typical shoulder indwelling and surface EMG signal. For the analysis at the narrow bandwidth (10–350 Hz), the percentage of total power spectrum contained in the frequency domain from 10 to 350 Hz was calculated and reported
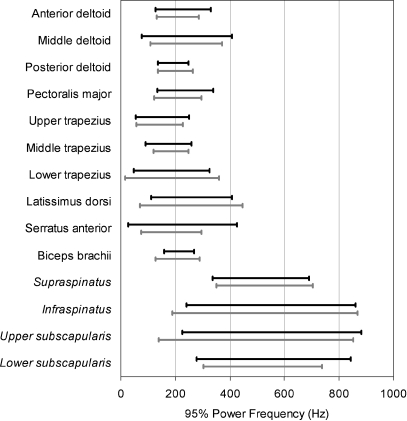
Fig. 3.
Two SDs about the mean for the ramp and plateau phases of the MVIC. The muscle names are italicized when an indwelling electrode was used for recording
Table 3.
Descriptive statistics, including mean and 2 SDs, were calculated of the 95% power frequency for both the ramp and the plateau subphases
| Muscle | Electrode | Ramp | Plateau | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (Hz) | 2 SDs | UL | Nyquist rate | 3× UL | Mean (Hz) | 2 SDs | UL | Nyquist rate | 3× UL | ||
| Anterior deltoid | Surface | 229 | 100 | 330 | 659 | 989 | 210 | 77 | 287 | 574 | 860 |
| Middle deltoid | Surface | 243 | 165 | 408 | 816 | 1,224 | 240 | 132 | 372 | 743 | 1,115 |
| Posterior deltoid | Surface | 193 | 56 | 249 | 499 | 748 | 200 | 64 | 264 | 527 | 791 |
| Pectoralis major | Surface | 237 | 104 | 340 | 681 | 1,021 | 209 | 86 | 296 | 592 | 887 |
| Upper trapezius | Surface | 152 | 98 | 250 | 500 | 750 | 143 | 85 | 228 | 455 | 683 |
| Middle trapezius | Surface | 176 | 83 | 259 | 518 | 777 | 185 | 64 | 249 | 498 | 747 |
| Lower trapezius | Surface | 187 | 138 | 325 | 650 | 975 | 188 | 172 | 360 | 719 | 1,079 |
| Latissimus dorsi | Surface | 260 | 149 | 408 | 817 | 1,225 | 258 | 188 | 446 | 893 | 1,339 |
| Serratus anterior | Surface | 226 | 199 | 425 | 851 | 1,276 | 186 | 110 | 295 | 591 | 886 |
| Biceps brachii | Surface | 214 | 55 | 269 | 537 | 806 | 208 | 81 | 290 | 580 | 869 |
| Supraspinatus | Indwelling | 514 | 177 | 691 | 1,382 | 2,074 | 527 | 177 | 703 | 1,407 | 2,110 |
| Infraspinatus | Indwelling | 551 | 309 | 860 | 1,721 | 2,581 | 528 | 340 | 868 | 1,735 | 2,603 |
| Upper subscapularis | Indwelling | 554 | 328 | 882 | 1,764 | 2,647 | 495 | 356 | 851 | 1,702 | 2,553 |
| Lower subscapularis | Indwelling | 560 | 283 | 844 | 1,687 | 2,531 | 521 | 217 | 737 | 1,475 | 2,212 |
The UL, Nyquist rate, and triple the UL (3× UL) are included. All values are in Hz
The indwelling EMG signals, shown in italics in Table 3, had more than twice the frequency content of the surface EMG signals. The ULs of the 95% power frequencies were all ≤882 Hz with indwelling electrodes and ≤446 Hz for surface electrodes.
A significant difference was found among muscles whose signals were recorded with surface electrodes (p = .002). Post hoc analysis Tukey B results are included in Table 4. No differences were found among muscles whose signals were recorded with indwelling electrodes (p = .961).
Table 4.
ANOVA (Tukey B) post hoc comparison for 95% power frequencies for EMG signals collected with surface electrodes
| Muscle | Subset for α = .05 | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Upper trapezius | 157.7 | ||
| Middle trapezius | 189.6 | 189.6 | |
| Lower trapezius | 198.0 | 198.0 | |
| Posterior deltoid | 206.7 | 206.7 | 206.7 |
| Serratus anterior | 229.6 | 229.6 | 229.6 |
| Biceps brachii | 229.7 | 229.7 | 229.7 |
| Anterior deltoid | 232.7 | 232.7 | 232.7 |
| Pectoralis major | 240.2 | 240.2 | 240.2 |
| Middle deltoid | 256.9 | 256.9 | |
| Latissimus dorsi | 285.3 | ||
Means for groups in homogeneous subsets are displayed
Significant differences were found when comparing the mean maximum 95% power frequencies recorded between electrode types (Table 5). No difference existed in the 95% power frequency between the subphases of contraction (ramp vs. plateau) for any muscle–electrode combination.
Table 5.
Comparison of the mean maximum 95% power frequency between surface to indwelling electrode with the paired sample statistics and the test of the paired differences
| Paired samples statistics | ||||||||
| Mean | N | SD | Standard error of the mean | |||||
| Average peak surface | 211.8 | 10 | 21.8 | 6.9 | ||||
| Average peak wire | 542.7 | 10 | 109.7 | 34.7 | ||||
| Paired samples test | ||||||||
| Paired differences | ||||||||
| Mean | SD | Standard error of the mean | 95% confidence interval difference | t | df | Significance (2 tailed) | ||
| Lower | Upper | |||||||
| Average peak surface − average peak wire | −330.9 | 114.9 | 36.3 | −413.1 | −248.7 | −9.1 | 9 | .000 |
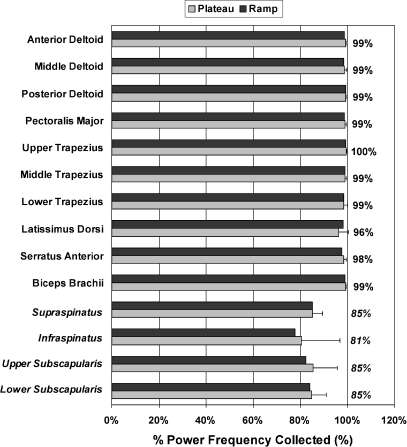
At the narrower bandwidth of 10 to 350 Hz, a minimum level of 95% power frequency was met for all of the surface muscles (Fig. 4). For muscles with indwelling electrodes, an 83 ± 3% power frequency was recorded at this narrower bandwidth setting.
Fig. 4.
The percentage of the power frequency (mean ± SD) for the ramp and plateau phases of the MVIC for narrower 10 to 350 Hz bandwidth condition data. The muscle names are italicized when an indwelling electrode was used for recording
Discussion
The frequencies of the EMG signal recorded with surface and indwelling electrodes are comparable to those documented in the lower extremity, cervical spine, and selected upper extremity muscles [23, 36]. The mean 95% power frequencies ranged from 152 to 260 Hz for surface electrodes and from 495 to 560 Hz for indwelling electrodes.
The decision to use the 95% level was reinforced as we proceeded through this project based on the display of data for each subject and for each muscle. The samples of typical data tracings of the power spectrum plots (power vs. frequency, Fig. 2) illustrate the common finding that at higher frequencies, the power was zero. The frequencies higher than this cutoff were interpreted to be higher frequency electrode and equipment noise.
By calculating the UL of the frequency of interest as the mean + 2 SDs of the 95% power frequency, we should encompass ≥95% of the population’s values. The highest Nyquist rate recorded was in the latissimus dorsi (893 Hz) for surface electrodes and in the upper subscapularis (1,764 Hz) for indwelling electrodes. These values represent the minimum frequency that should be selected if the entire spectrum is the outcome measure of interest. If, however, we follow the ISEK recommendation of sampling EMG greater than twice the frequency of interest and the more conservative value of triple—not double—the UL, then shoulder muscles studied with surface EMG should be sampled at ≥1,340 Hz. Shoulder muscles studied with indwelling EMG should be sampled at ≥2,650 Hz.
The higher frequencies measured with the use of indwelling electrodes as compared to surface electrodes are consistent with ISEK standards as well as previous literature [26, 28, 35, 38]. Within the rotator cuff muscles, all with indwelling electrodes, no systematic or significant differences in the 95% power frequencies were recorded regardless of subphase. This was not the case in the muscles where surface electrodes were used. The 95% power frequency was lower for all three parts of the trapezius as compared to the latissimus dorsi. The trapezius, with its direct attachment to the spine, acts both as a postural support muscle and an active shoulder mover, and thus, the lower firing rate is consistent with the firing rates in cervical muscles [22]. For the muscles tested with surface electrodes whose 95% power frequencies were in the mid range (posterior deltoid, serratus anterior, biceps brachii, anterior deltoid, and pectoralis major), no differences were found among these muscles. Unless individual sampling rates are used, the overall guideline of 1,340 Hz frequency will still hold for this group when data are recorded with surface electrodes. Like the lower extremity, choice of electrode (surface or indwelling) appears to outweigh the difference among muscles when the frequency content of the recorded EMG signal is the variable of interest [28].
There may be something unique about the rotator cuff muscles, and they might have continued to have higher frequencies even if a surface electrode was used. However, the choice of electrode was more likely the determining factor. It should be pointed out that this question could only be decidedly tested if both surface and fine wire electrodes were used on the muscles simultaneously, which was not, however, our main purpose.
EMG signals were recorded only during MVICs. In previous work with surface EMGs, the power spectrum shifted to lower frequencies when muscles were fatigued [8, 9, 13]. Also, as compared to concentric contractions, a similar shift in the power spectrum to lower frequencies occurred when eccentric contractions were performed [24]. Finally, even during contractions at various intensity levels, no significant change in the spectral characteristics was noted despite changes in signal amplitude [9, 21]. Thus, these sampling recommendations should not be sensitive to fatigue, type of contraction, or intensity level.
No differences were observed in the 95% power frequencies for a given muscle–electrode combination based on the subphase of contraction (ramp vs. plateau). This implies that, despite the increasing force of contraction as a subject performs an isometric contraction, the Nyquist rates established here are sufficiently high to capture data regardless of the subphase. The higher signal amplitude during plateau did not correspond to a higher signal frequency.
The narrower bandwidth analysis confirms that if researchers and clinicians are interested in the higher frequency portion of the EMG signal measured with indwelling electrodes, a higher sampling rate is required. With surface electrodes, no loss of frequency content occurred in the EMG signal. For indwelling electrodes, a consistent loss of higher frequency content of ~15% was found. These results do not invalidate previous studies with surface electrodes, low-pass filtering, or decreased sampling rates if the range of frequencies of interest was lower.
Undersampling remains a potential concern for all signal recording. Researchers and clinicians should take advantage of improved technology, including increased computing power and data storage, by increasing the sampling rates for routine data collection of shoulder EMGs. As a conservative estimate to eliminate the risk of undersampling when using surface EMGs, shoulder muscles should be sampled at a minimum of 1,340 Hz; when indwelling electrodes are used, shoulder muscles should be sampled at a minimum of 2,650 Hz.
Acknowledgements
The authors gratefully acknowledge Rebecca A. Zifchock, Ph.D., and Timothy M. Wright, Ph.D., for their assistance with manuscript preparation.
Footnotes
Financial Disclosure: Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Ethical Review: Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
Level of Evidence: Four Case Series
References
- 1.European Recommendations for Surface Electromyography: results of the SENIAM project. Enschede, the Netherlands: Roessingh Research and Development; 1999.
- 2.Beck TW, Housh TJ, Johnson GO, et al. The effects of interelectrode distance on electromyographic amplitude and mean power frequency during isokinetic and isometric muscle actions of the biceps brachii. J Electromyogr Kinesiol. 2005;15:482–495. doi: 10.1016/j.jelekin.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Bilodeau M, Arsenault AB, Gravel D, et al. The influence of an increase in the level of force on the EMG power spectrum of elbow extensors. Eur J Appl Physiol Occup Physiol. 1990;61:461–466. doi: 10.1007/BF00236068. [DOI] [PubMed] [Google Scholar]
- 4.Bogey R, Cerny K, Mohammed O. Repeatability of wire and surface electrodes in gait. Am J Phys Med Rehabil. 2003;82:338–344. doi: 10.1097/00002060-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Cordasco FA, Chen NC, Backus SI, et al. Subacromial injection improves deltoid firing in subjects with large rotator cuff tears. HSS J. 2010;6:30–36. [DOI] [PMC free article] [PubMed]
- 6.Cordasco FA, Wolfe IN, Wootten ME, et al. An electromyographic analysis of the shoulder during a medicine ball rehabilitation program. Am J Sports Med. 1996;24:386–92. doi: 10.1177/036354659602400323. [DOI] [PubMed] [Google Scholar]
- 7.Decker MJ, Tokish JM, Ellis HB, et al. Subscapularis muscle activity during selected rehabilitation exercises. Am J Sports Med. 2003;31:126–134. doi: 10.1177/03635465030310010601. [DOI] [PubMed] [Google Scholar]
- 8.Dimitrova NA, Dimitrov GV. Interpretation of EMG changes with fatigue: facts, pitfalls, and fallacies. J Electromyogr Kinesiol. 2003;13:13–36. doi: 10.1016/S1050-6411(02)00083-4. [DOI] [PubMed] [Google Scholar]
- 9.Duchene J, Goubel F. EMG spectral shift as an indicator of fatigability in an heterogeneous muscle group. Eur J Appl Physiol Occup Physiol. 1990;61:81–87. doi: 10.1007/BF00236698. [DOI] [PubMed] [Google Scholar]
- 10.Durkin JL, Callaghan JP. Effects of minimum sampling rate and signal reconstruction on surface electromyographic signals. Journal of Electromyography and Kinesiology. 2005;15:474–481. doi: 10.1016/j.jelekin.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Farina D, Fosci M, Merletti R. Motor unit recruitment strategies investigated by surface EMG variables. J Appl Physiol. 2002;92:235–247. doi: 10.1063/1.1481974. [DOI] [PubMed] [Google Scholar]
- 12.Fuglsang-Frederiksen A, Rønager J. The motor unit firing rate and the power spectrum of EMG in humans. Electroencephalogr Clin Neurophysiol. 1988;70:68–72. doi: 10.1016/0013-4694(88)90196-4. [DOI] [PubMed] [Google Scholar]
- 13.Gerdle B, Larsson B, Karlsson S. Criterion validation of surface EMG variables as fatigue indicators using peak torque: a study of repetitive maximum isokinetic knee extensions. J Electromyogr Kinesiol. 2000;10:225–232. doi: 10.1016/S1050-6411(00)00011-0. [DOI] [PubMed] [Google Scholar]
- 14.Giroux B, Lamontagne M. Comparisons between surface electrodes and intramuscular wire electrodes in isometric and dynamic conditions. Electromyogr Clin Neurophysiol. 1990;30:397–405. [PubMed] [Google Scholar]
- 15.Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- 16.Ives JC, Wigglesworth JK. Sampling rate effects on surface EMG timing and amplitude measures. Clin Biomech. 2003;18:543–552. doi: 10.1016/S0268-0033(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 17.Kaplanis PA, Pattichis CS, Hadjileontiadis LJ, et al. Surface EMG analysis on normal subjects based on isometric voluntary contraction. J Electromyogr Kinesiol. 2009;19:157–171. doi: 10.1016/j.jelekin.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Kelly BT, Backus SI, Warren RF, et al. Electromyographic analysis and phase definition of the overhead football throw. Am J Sports Med. 2002;30:837–844. doi: 10.1177/03635465020300061401. [DOI] [PubMed] [Google Scholar]
- 19.Kelly BT, Cooper LW, Kirkendall DT, et al. Technical considerations for electromyographic research on the shoulder. Clin Orthop Relat Res. 1997;335:140–151. [PubMed]
- 20.Kelly BT, Williams RJ, 3rd, Cordasco FA, et al. Differential patterns of muscle activation in patients with symptomatic and asymptomatic rotator cuff tears. J Shoulder Elbow Surg. 2005;14:165–171. doi: 10.1016/j.jse.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Komi PV, Linnamo V, Silventoinen P, et al. Force and EMG power spectrum during eccentric and concentric actions. Med Sci Sports Exerc. 2000;32:1757–1762. doi: 10.1097/00005768-200010000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Kumar S, Narayan Y, Amell T. EMG power spectra of cervical muscles in lateral flexion and comparison with sagittal and oblique plane activities. Eur J Appl Physiol. 2003;89:367–376. doi: 10.1007/s00421-003-0797-3. [DOI] [PubMed] [Google Scholar]
- 23.Kumar S, Narayan Y, Amell T. Power spectra of sternocleidomastoids, splenius capitis, and upper trapezius in oblique exertions. Spine J. 2003;3:339–350. doi: 10.1016/S1529-9430(03)00077-9. [DOI] [PubMed] [Google Scholar]
- 24.Moritani T, Muramatsu S, Muro M. Activity of motor units during concentric and eccentric contractions. Am J Phys Med. 1987;66:338–350. [PubMed] [Google Scholar]
- 25.Moritani T, Muro M. Motor unit activity and surface electromyogram power spectrum during increasing force of contraction. Eur J Appl Physiol Occup Physiol. 1987;56:260–265. doi: 10.1007/BF00690890. [DOI] [PubMed] [Google Scholar]
- 26.Oberg T, Sandsjo L, Kadefors R. Arm movement and EMG mean power frequency in the trapezius muscle: a comparison between surface and intramuscular recording techniques. Electromyogr Clin Neurophysiol. 1992;32:87–96. [PubMed] [Google Scholar]
- 27.Oppenheim AV, Schafer RW. Digital signal processing. US Ed ed. Englewood Cliffs, NJ: Prentice-Hall, Inc; 1975.
- 28.Perry J, Easterday CS, Antonelli DJ. Surface versus intramuscular electrodes for electromyography of superficial and deep muscles. Phys Ther. 1981;61:7–15. doi: 10.1093/ptj/61.1.7. [DOI] [PubMed] [Google Scholar]
- 29.Pullman SL, Goodin DS, Marquinez AI, et al. Clinical utility of surface EMG: report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology. 2000;55:171–177. doi: 10.1212/wnl.55.2.171. [DOI] [PubMed] [Google Scholar]
- 30.Riley ZA, Terry ME, Mendez-Villanueva A, et al. Motor unit recruitment and bursts of activity in the surface electromyogram during a sustained contraction. Muscle Nerve. 2008;37:745–753. doi: 10.1002/mus.20978. [DOI] [PubMed] [Google Scholar]
- 31.Sadhukhan AK, Goswami A, Kumar A, et al. Effect of sampling frequency on EMG power spectral characteristics. Electromyogr Clin Neurophysiol. 1994;34:159–163. [PubMed] [Google Scholar]
- 32.Schweitzer TW, Fitzgerald JW, Bowden JA, et al. Spectral analysis of human inspiratory diaphragmatic electromyograms. J Appl Physiol. 1979;46:152–165. doi: 10.1152/jappl.1979.46.1.152. [DOI] [PubMed] [Google Scholar]
- 33.Sleigh JW, Donovan J. Comparison of bispectral index, 95% spectral edge frequency and approximate entropy of the EEG, with changes in heart rate variability during induction of general anaesthesia. Br J Anaesth. 1999;82:666–671. doi: 10.1093/bja/82.5.666. [DOI] [PubMed] [Google Scholar]
- 34.Soames RW, Atha J. The spectral characteristics of postural sway behaviour. Eur J Appl Physiol Occup Physiol. 1982;49:169–177. doi: 10.1007/BF02334065. [DOI] [PubMed] [Google Scholar]
- 35.Soderberg GL, Knutson LM. A guide for use and interpretation of kinesiologic electromyographic data. Phys Ther. 2000;80:485–98. [PubMed] [Google Scholar]
- 36.Tesch PA, Komi PV, Jacobs I, et al. Influence of lactate accumulation of EMG frequency spectrum during repeated concentric contractions. Acta Physiol Scand. 1983;119:61–67. doi: 10.1111/j.1748-1716.1983.tb07306.x. [DOI] [PubMed] [Google Scholar]
- 37.Turker KS. Electromyography: some methodological problems and issues. Phys Ther. 1993;73:698–710. doi: 10.1093/ptj/73.10.698. [DOI] [PubMed] [Google Scholar]
- 38.Winter DA, Rau G, Kadefors R, et al. Units, terms and standards in the reporting of EMG research. Report by the As Hoc Committee of ISEK. 1980; Aug.