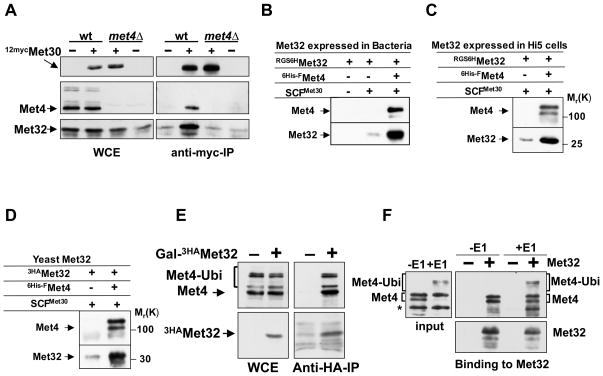
Figure 2. Met4 recruits Met32 to the SCFMet30 ubiquitin ligase in vivo and in vitro.
(A) In vivo interaction between Met30 and Met32 depends on Met4.
Wild-type cells and met4Δ mutants expressing endogenous 12mycMet30 and GAL1-3HAMet32 were grown in galactose medium containing 1mM. 12mycMet30 was immunopurified from total cell lysates and immunocomplexes were analyzed with anti-myc, anti-Met4, and anti-HA antibodies. Wild-type cells and met4Δ mutants expressing untagged Met30 were processed in parallel as controls.
(B and C) In vitro binding of recombinant Met32 expressed in E. coli and insect cells. Immobilized SCFMet30 and SCFMet30/Met4 complexes produced in insect cells were incubated with RGS6HMet32 expressed in E. coli (panel B), or insect cells (panel C). Bound proteins were eluted and analyzed by immunoblotting using anti-Met4 and anti-RGS4His antibodies.
(D) In vitro binding of Met32 expressed in yeast. Experiment as in panel B, except that 3HAMet32 expressed in yeast cells (met4Δ met30Δ) was used.
(E) Met32 binds to ubiquitylated and de-ubiquitylated Met4 in vivo. 3HAMet32 was immunopurified from yeast cells and immunocomplexes were analyzed by Western blotting using anti-HA and anti-Met4 antibodies. Cells expressing untagged Met32 were used as control.
(F) Met32 binds to ubiquitylated and de-ubiquitylated Met4 in vitro. Recombinant Met4 was bound to recombinant SCFMet30 and ubiquitylated in vitro to generate SCFMet30/Met4 containing ubiquitylated Met4. The same complex but containing non-ubiquitylated Met4 was obtained by omitting E1 from the reaction. Both complexes were incubated with immobilized RGS6His-3HAMet32 purified from E. coli. Bound fractions were analyzed by immunoblotting with anti-HA and anti-Met4 antibodies. Anti-HA beads without RGS6His-3HA Met32 were used as control.