Abstract
Myelination in the peripheral nervous system (PNS) is induced by close contact signaling between axons and Schwann cells. Previous studies have identified membrane-bound neuregulin-1 (Nrg1) type III, expressed on the axons, as the key instructive signal that regulates Schwann cell myelination. In our recent study, we show that recombinant soluble Nrg1 elicits a similar pro-myelinating effect on Schwann cells, albeit in a dosage-dependent manner: Nrg1 promotes myelination at low concentrations but inhibits it at high concentrations. The inhibitory effect of Nrg1 is mediated through its activation of the Ras/Raf/Erk pathway in Schwann cells, and inhibition of the pathway using a pharmacologic inhibitor restores myelination. We also show that soluble Nrg1 enhances myelination on axons that do not express sufficient amount of Nrg1 type III needed for robust myelination. These findings are significant as they suggest that combined therapies aimed at enhancing Nrg1 signaling and blocking the Ras/Raf/Erk activation may be an effective strategy for improving remyelination on adult axons, which, as shown in our recent data, express low levels of Nrg1 type III. In this report we provide an overview of our recent findings and discuss the therapeutic potential of soluble Nrg1.
Keywords: Remyelination, Ras/Raf/Erk, PI3-kinase, Adult axons, ErbB
Introduction
The formation of myelin enables saltatory conduction required for rapid and efficient propagation of action potentials in the nervous system. Loss of myelin has been implicated in various neurodegenerative diseases including multiple sclerosis, Charcot-Marie-Tooth disease, Guillian-Barre syndrome, neuropathies secondary to diabetes and cancer chemotherapy and infectious neuropathies. Because of the intimate connection between the myelin-forming glial cells and the axons, demyelination is also linked to progressive loss of neuronal function. Therefore, rebuilding myelin in demyelinated lesions is an important therapeutic objective for addressing neurodegenerative diseases.
The molecular mechanisms that regulate myelination remain elusive. In the peripheral nervous system (PNS), the process begins with Schwann cells, the myelin-forming glial cells, forming a 1:1 association with axons to be myelinated. As a Schwann cell wraps around an axon, one end of the Schwann cell membrane slips under the other and extends forward as it encircles the axon. The membrane continues to extend, forming multiple concentric membrane lamellae, which later compact to form the myelin sheath. The molecular signal that triggers Schwann cell myelination is provided by the axon with which the Schwann cell associates. Specifically, the type III isoform of neuregulin-1 (Nrg1) expressed on PNS axons has been shown to play a key role in regulating myelination (1, 2).
The Nrg1 growth factor family includes type I (ARIA, heregulin, and NDF), type II (GGF) and type III (SMDF) isoforms. These are derived from one gene and generated by use of multiple transcription sites and by extensive alternative RNA splicing (3). The type I, II and III isoforms are characterized by differences in their N-terminal segments. All isoforms contain an epidermal growth factor (EGF)-like signaling domain that is necessary and sufficient for activation of erbB receptors (4, 5). In addition to the EGF-like domain, Nrg1 type I contains an Ig-like domain and a glycosylation-rich segment (4). Type II isoforms also contain an Ig-like domain but lack the glycosylation-rich segment (6). Type III isoforms lack both the Ig-like domain and the glycosylation-rich segment but contain a cystein-rich domain (CRD), which functions as a second transmembrane domain (7). Type I and II isoforms, which are synthesized as transmembrane precursor proteins, undergo cleavage near the transmembrane region and are released as soluble forms, thus function as paracrine signaling molecules (8). On the other hand, due to its unique CRD domain, type III Nrg1 remains tethered to the cell surface after cleavage and function as a juxtacrine signal (9). Neuregulin-1 expression is found mostly on CNS and PNS neurons and accordingly, has been shown to play important roles during development of the nervous system, including that of the Schwann cell lineage and PNS myelination (10).
Schwann cell development and myelination depend on contact-mediated signaling between Schwann cells and the associated PNS axons. The Nrg1-erbB ligand-receptor system lends itself to the task of close contact signaling, as the axons express Nrg1 family ligands and the Schwann cells express erbB2 and erbB3, which dimerize upon binding to ligand to form functional Nrg1 receptors. Studies using transgenic and conditional knockout mice have established that the Nrg1 type III mediated erbB receptor signaling plays an essential role during Schwann cell myelination (11). For example, analysis of mice with a conditional erbB2 knockout in prenatal Schwann cells showed that erbB2 signaling is necessary for proper myelin formation (12, 13). Analysis of Nrg1 type III knockout and transgenic mice showed that the axonal Nrg1 isoform is not only required for Schwann cell myelination, but also serves as a key instructive signal that determines the myelination state of the PNS axons (1, 2).
In contrast to the pro-myelinating function of Nrg1 type III, GGF, a type II isoform, has been shown to play a negative role during myelination. Treatment of Schwann cells with GGF inhibits myelination in cultures (14). In vivo expression of GGF transgene results in PNS demyelination and increased Schwann cell proliferation (15). Since both type II and type III isoforms bind and activate the same erbB receptor complex on the Schwann cell surface, it is unclear how they elicit two opposing effects on myelination. It has been suggested that myelination requires activation of the erbB signal at the Schwann cell-axon junction, induced by the membrane-bound axonal Nrg1 type III signal whereas paracrine activation of the receptors by soluble Nrg1 such as GGF, inhibits myelination (2, 11). It is also possible that type II and type III Nrg1 differentially regulate the erbB receptor downstream signaling pathways that promote or inhibit myelination.
In our recent study, we addressed the issue by investigating the signaling property of soluble Nrg1 and the effect on Schwann cell myelination (16). Herein, we present an overview of our findings and discuss the therapeutic potential of soluble Nrg1 in promoting remyelination of adult axons.
Materials and Methods
Antibodies
For Western blot analysis, monoclonal antibody to phospho-Akt (Cell signaling, Danvers, MA) and polyclonal antibody to phospho-Erk1/2 (Promega, Madison, WI) were used at 1:1000 and 1:5000, respectively. Polyclonal antibodies to Akt (Cell Signaling, Danvers, MA) and Erk1/2 (Promega, Madison, WI) were used at 1:1000 and 1:5000, respectively. Polyclonal antibodies to erbB2 and phospho-erbB2 were used at 1:500 (Santa Cruz, Biotech, Santa Cruz, CA). Monoclonal antibody to β-actin (Sigma-Aldrich, St. Louis, MO) was used at 1:5000. Polyclonal antibody to Nrg1-type III (Santa Cruz Biotech, Santa Cruz, CA) was used at 1:500.
Preparation of embryonic dorsal root ganglion (DRG) neuron cultures
Dissociated DRGs were prepared from embryonic day (E) 15.5 rat embryos as described previously (Eldridge et al., 1987) and plated onto Matrigel-coated 12 mm glass coverslips at a density of 0.8 DRG per coverslip. Briefly, DRGs were dissociated in 0.25% Trypsin for 30 minutes at 37°C and plated onto coverslips in DMEM (Mediatech, Herndon, VA) supplemented with 10% FBS (Mediatech, Manassas, VA), 50 ng/ml of NGF (Harlan Bioproducts, Indianapolis, Indiana) in a 130 µl droplet. Five to six hours later, the cultures were flooded with Neurobasal medium (Mediatech, Manassas, VA) supplemented with B27 (GIBCO, Carlsbad, CA), 0.08% glucose, NGF (50 ng/ml) and a mixture of 15 µM 5-fluorodeoxyuridine (FdUr) (Sigma-Aldrich, St. Louis, MO) and Uridine (Sigma-Aldrich, St.Louis, MO). Cultures were maintained in the same medium for two days to remove proliferating non-neuronal cells then switched to fresh medium without the FdUr-Uridine mixture. Cycling in FdUr-Uridine was continued for 10 days until all non-neuronal cells were removed and the DRG axons reached the periphery of the coverslips.
Preparation of adult DRG neuron cultures
The Dissociated DRG neurons were prepared from adult rats as described previously with modification (17). Briefly, the spinal column of an adult rat was removed and incisions were made along the ventral and the dorsal surface to expose DRGs. The DRGs were removed and collected in a 100-mm Petri dish containing L-15 media (Invitrogen, Carlsbad, CA). After trimming off the attached nerve strings, DRGs were dissociated in 0.25% collagenase for 2 hours at 37°C then dissociated mechanically by trituration using a narrow-bore glass Pasteur pipette. The Myelin debris and endogenous Schwann cells were removed by passing the dissociated DRGs through two layers of BSA gradient (5% and 10%) twice by centrifugation at 115xg for 4 minutes. The pellet containing DRGs were then suspended in Neurobasal medium supplemented with B27, 0.08% glucose and 50 ng/ml of NGF (Harlan Bioproducts, Indianapolis, Indiana) and plated onto Matrigel-coated 12 mm glass coverslips at a density of 2.5 DRGs per coverslip. Five to six hours later, the cultures were flooded with Neurobasal medium (Mediatech, Manassas, VA) with supplements as above and 15 µM 5-fluorodeoxyuridine (FdUr) (Sigma-Aldrich, St. Louis, MO) and Uridine (Sigma-Aldrich, St.Louis, MO). Cultures were maintained in the medium for 6–7 days until the non-neuronal cells were removed and the axons extended out to the periphery of the coverslips.
Preparation of neurite membrane fraction from neuron cultures
Neurite membrane fractions were prepared as described previously (Maurel and Salzer 2000). Briefly, dissociated DRG neurons were prepared as described above in 35mm culture dishes until the axons extend out to the periphery. Neurons were scraped off the plate, homogenized in PBS using a Dounce homogenizer (Wheaton, USA) and centrifuged at 80xg for 20 minutes at 4°C to remove any cell debris and collagen. The supernatant was collected and the membrane fractions were collected by ultracentrifugation at 35,000xg for 1 hour at 4°C. After determining the protein concentrations, an equal amount of adult and embryonic neurite membrane fractions were centrifuged (200 X g, 10 minutes, 4°C) onto serum starved rat primary Schwann cells. After incubating at 37°C for 20 minutes, cell lysates were prepared, size fractionated on SDS-PAGE and then subjected to Western blot analysis.
Western blot analysis
Cultures were washed in PBS and lysed in ice-cold buffer containing 20 mM Tris HCl pH7.4, 1% NP-40, 10% Glycerol, 2.5 mM EGTA, 2.5 mM EDTA, 150 mM NaCl, 20 µM leupeptin, 10µg/ml aprotinin, 1 mM phenylmethane sulphonyl fluoride (PMSF) and 1 mM sodium orthovanadate. Lysates were centrifuged for 15 min at 14,000 rpm at 4°C, supernatants were collected and the protein concentrations were determined using the Bradford method. Twenty micrograms of lysates were size-fractionated on a 10% SDS-polyacrylamide gel and transferred onto a PVDF membrane. After blocking in 5% milk for 1 hour, membranes were incubated with the appropriate primary antibodies. Membranes were washed three times in TBST and incubated with HRP-conjugated secondary antibodies. The protein bands were visualized by enhanced chemiluminescence (ECL, Pierce) and quantified using Image J software.
Results and Discussion
The pro-myelinating function of soluble neuregulin-1
Previous studies have shown that soluble Nrg1 such as GGF (type II) inhibits Schwann cell myelination (14) whereas the membrane-bound Nrg1 type III promotes myelination (1, 2); this has led to the notion that the promyelinating function of Nrg1 type III is mediated by juxtacrine signaling activated at the Schwann cell-axon junction. To further investigate the signaling function of Nrg1 type III, we asked in our recent study (16) whether the Nrg1 type III presented in a paracrine manner would promote or inhibit Schwann cell myelination. Our data showed that unlike GGF, soluble Nrg1 type III promoted myelination by increasing the number of myelin segments formed in Schwann cell-DRG neuron co-cultures. Furthermore, treatment with soluble Nrg1 increased internodal lengths of individual segments, indicating that the ectopic Nrg1 signal also promotes myelin maturation. This is an important finding since it suggests a therapeutic potential of soluble Nrg1 type III in promoting remyelination in the PNS, which often results in formation of immature myelin segments.
One of the intriguing findings of our study was the concentration-dependent biphasic effect of soluble Nrg1 on Schwann cell myelination, which was independent of the Nrg1 isoforms. Our data showed that while soluble Nrg1 type III promoted myelination at concentrations ranging from 0.1 to 1 nM, it began to inhibit myelination when dosages was increased. A similar dose-dependent, biphasic effect was observed with GGF, previously regarded as a negative regulator of Schwann cell myelination. Biochemical analysis revealed that the inhibitory effect of soluble Nrg1 observed at high concentrations coincided with the appearance of active Mek/Erk. On the other hand, activation of the PI3-kinase pathway was induced early on at low concentrations and persisted throughout. This result indicates a dose-dependent differential activation of the Nrg1 signaling in Schwann cells. When cultures were treated with Mek/Erk inhibitor, the inhibitory effect of Nrg1 was reversed, confirming that the Nrg1 function was mediated through its activation of the Ras/Raf/Erk pathway. This is in agreement with a previous study, which has shown that growth factor induced Mek/Erk activation inhibits Schwann cell differentiation (18). Our data also provide direct evidence supporting the role of the Ras/Raf/Erk pathway as a negative regulator of Schwann cell myelination. Altogether, these results show that the paracrine signal of soluble Nrg1 exhibits two contrasting effects on Schwann cell myelination and the effects are determined by the dose-dependent differential activation of the Ras/Raf/Erk pathway.
We also showed that inhibition of the Mek/Erk activity endogenous to the co-cultures increased myelination significantly. This result indicates the presence of an intrinsic Mek/Erk-dependent signal that functions as a negative regulator of Schwann cell myelination. The nature of the signal that activates Mek/Erk in myelinating Schwann cell is unknown. The signal is likely to be axonal in origin however, distinct from the Nrg1 type III signal, which has been shown to be the key neuronal signal that activates the PI3-kinase but not the Ras/Raf/Erk pathway in the associated Schwann cells (2). The PNS neurons express other growth factors such as FGF-2 and PDGF that are capable of activating the Ras/Raf/Erk pathway and Schwann cells express the corresponding receptors (19–22). Furthermore, FGF-2 has been shown to inhibit myelination in Schwann cell-DRG co-cultures (14). It will be of great interest to assess the role of these growth factors in regulating myelination in the PNS.
The mechanism by which low doses of soluble Nrg1 promotes myelination is unclear. Previous studies have established the importance of the PI3-kinase activation during Schwann cell myelination. For example, constitutive activation of Akt in Schwann cells results in hyper-myelination (18) whereas inhibition of the PI3-kinase activity blocks myelination (23). We showed that at low concentrations, soluble Nrg1 activates the PI3-kinase but not the Ras/Raf/Erk pathway. Therefore, the preferential activation of the PI3-kinase is likely to contribute to the pro-myelinating effect of soluble Nrg1 at low concentrations.
Axons of the DRG neurons prepared from Nrg1 type III+/− mice are thinly myelinated and show reduced ability to activate PI3-kinase in the associated Schwann cells (2). We showed that treatment with soluble Nrg1 enhances myelination on these axons, along with an increase in PI3-kinase activation and expression of Krox 20, a transcription factor required for the development of the myelinating Schwann cell lineage. Interestingly however, in the complete absence of the axonal Nrg1 type III expression, soluble Nrg1 fail to induce myelination as we have shown in Nrg1 type III−/− co-cultures. This result suggests the exclusive requirement of the juxtacrine Nrg1 signal in initiating myelination. It is likely that the juxtacrine Nrg1-erbB signal at the Schwann cell-axon junction is required for the early events of myelination, which may include axon-Schwann cell association, axon-segregation and ensheathment or establishment of the initial Schwann cell polarity prior to myelination. Once these initial events are completed, paracrine stimulation of Nrg1 is sufficient to promote the subsequent events of myelination.
In the PNS, small diameter axons are normally unmyelinated but ensheathed by the Schwann cells whereas large diameter axons are heavily myelinated. A previous study has shown that the myelination state of the PNS axon is determined by the amount of Nrg1 type III expressed, which is proportional to the axonsize (2). This was demonstrated in an experiment in which forced expression of Nrg1 type III in sympathetic neurons of superior cervical ganglia (SCG) converted the normally unmyelinated axons to myelinated ones. A significant finding of our study is that soluble Nrg1 mimics this instructive role of axonal Nrg1 type III on myelination. We showed that treatment with soluble Nrg1 type III was sufficient to induce myelination on normally unmyelinated axons of the SCG neurons. This result further strengthens the pro-myelinating role of soluble Nrg1.
The pro-myelinating effect of Nrg1 may not be limited to the PNS since a recent study has shown that soluble Nrg1 also promotes myelination by oligodendrocytes, the myelin-forming glial cells of the CNS (36). Accordingly, it has been shown that CNS neurons express Nrg1 and oligodendrocytes express erbB receptors (25). Studies using transgenic mouse models have shown that although the Nrg1-erbB signaling is not required for myelination in the CNS, increasing the expression of Nrg1 type III is sufficient to promote oligodendrocyte myelination, resulting in hypermyelination in vivo (24).
The therapeutic potential of soluble neuregulin-1 for improving remyelination on adult axons
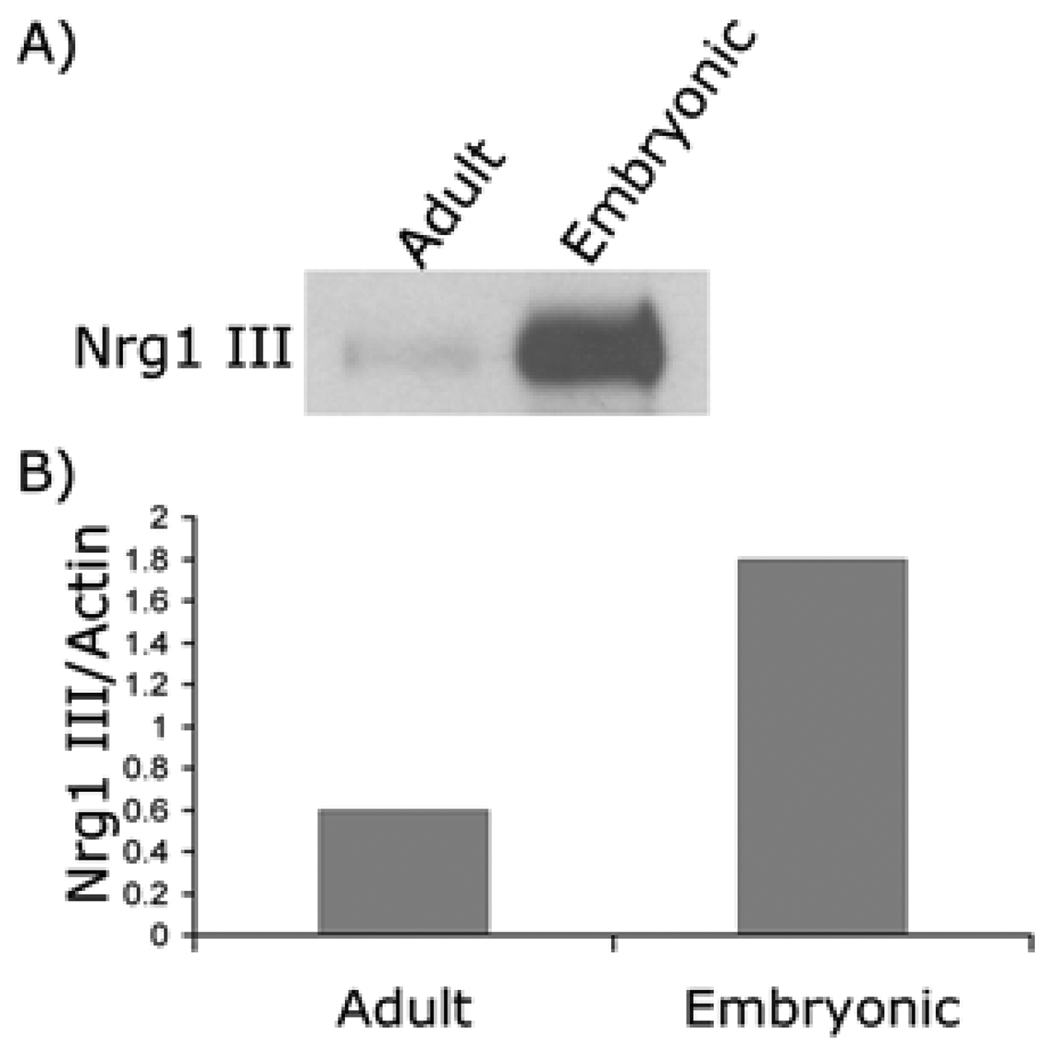
Experimental transplantation of myelinating glial cells has provided overwhelming proof for their potential at repairing damaged nerves. Schwann cells are good candidates for such therapy (26) as they can be easily expanded in culture when isolated from adult human peripheral nerves (27) and grafted in nerve lesion, thus offering the possibility of autologous transplantation into humans to promote remyelination and restore nerve conduction (28–33). Despite the benefits, however, remyelination on adult axons by transplanted Schwann cells is often incomplete, resulting in intermittent demyelinated areas and formation of myelin segments that are short and thin (34, 35). It is unclear why adult or regenerating axons do not become fully myelinated. Since Nrg1 type III plays a key role in regulating Schwann cell myelination, we speculated that mature adult axons might express insufficient levels of Nrg1 type III required for myelination. Supporting this notion, a previous study has shown that the expression levels of Nrg1 type III in the PNS decreases dramatically in postnatal animals (2). To investigate this, we prepared neuron cultures from embryonic and adult rat DRG and compared the Nrg type III expression levels by Western blot analysis. As shown in Figure 1, adult DRG neurons appeared to express lower level of Nrg1 type III compared to the embryonic neurons. Quantitative Western blot analysis showed that the amount of Nrg1 type III expressed in adult neurons was three-fold lower than that of embryonic neurons.
Figure 1. Expression of Nrg1 type III in adult and embryonic neurons.
(A) Cell lysates were prepared from adult and embryonic neuron cultures and subjected to Western blotting. The expression of Nrg1 type III was reduced in adult compared to embryonic neurons. (B) Quantification of the result shown in (A).
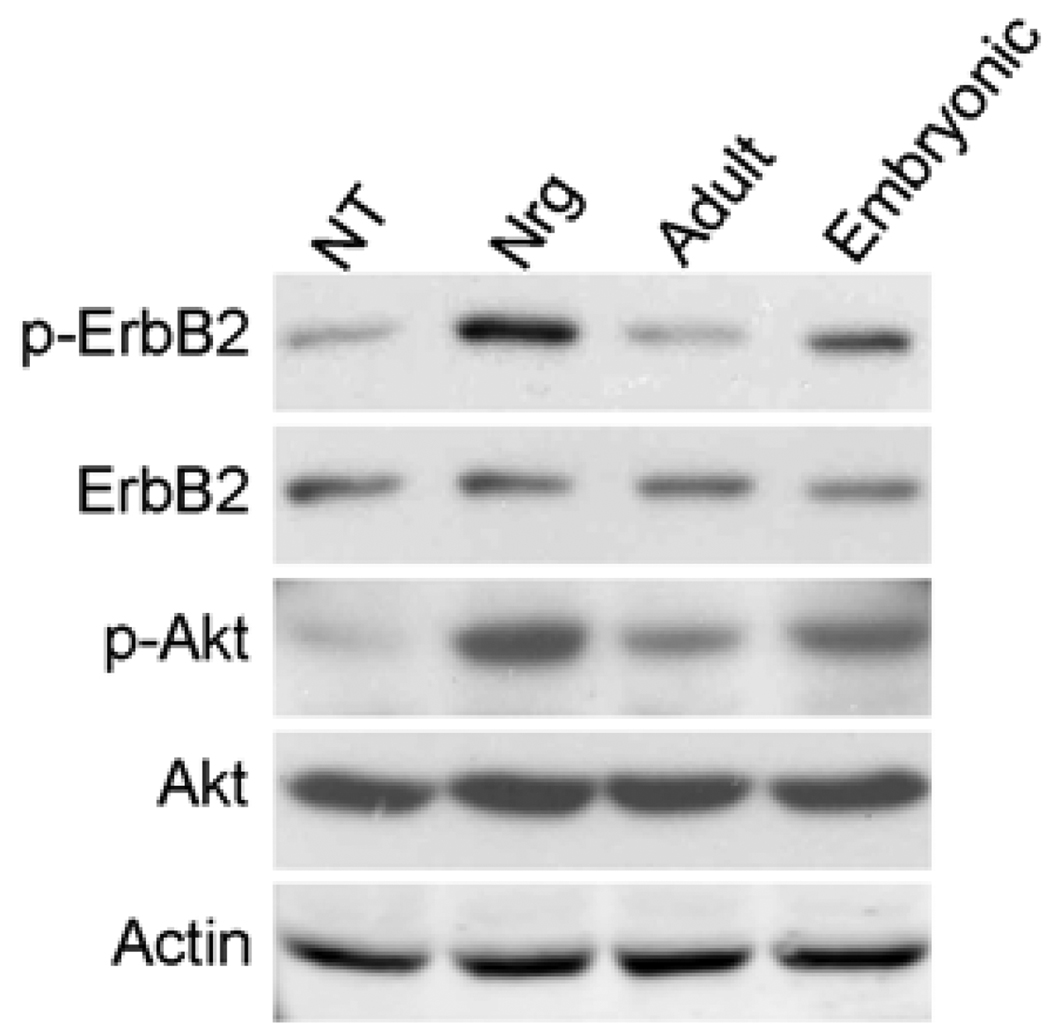
Axonal Nrg1 type III is the key activator of PI3-kinase in Schwann cell that is crucial for myelination (2, 18, 23). Therefore, we determined whether the low levels of Nrg1 type III expressed on adult axons affected erbB2 and the PI3-kinase activation in Schwann cells. We prepared membrane fractions from embryonic and adult DRG neurons and plated them onto Schwann cells for 20 minutes and then measured the activation levels of erbB2 and Akt. As shown in Figure 2, membranes from adult neurons had a decreased ability to activate erbB2 as well as Akt. Accordingly, we also observed that the level of Schwann cell myelination was also drastically decreased in adult DRG-Schwann cell co-cultures (data not shown). These results suggest that adult axons do not provide sufficient pro-myelinating signals to the associated Schwann cells due to the low expression levels of Nrg1 type III. The result also suggests a therapeutic potential of soluble Nrg1 to improve remyelination on adult axons by increasing the erbB-PI3-kinase activation through systemic or local delivery of the Nrg1 to the site of Schwann cell transplantation or demyelination. However, it should be cautioned that the therapeutic application could have adverse effects if administered at high concentrations as discussed above, thus precise dosing of Nrg1 would be crucial to maximize the therapeutic effect. Furthermore, development of a combined strategy to inhibit signals that negatively regulate myelination, such as the Ras/Raf/Erk pathway, will increase the effectiveness of the treatment for promoting remyelination.
Figure 2. Activation of ErbB2 and Akt in Schwann cells induced by membrane fractions prepared from adult and embryonic neurons.
Neurite membrane fractions were prepared from adult and embryonic neurons and added onto Schwann cells. After 20 minutes, cell lysates were prepared and subjected to Western blot analysis. Control Schwann cell cultures were treated with soluble Nrg1. NT: no treatment. The levels of erbB2 and Akt activation are much lower in Schwann cells treated with adult neurite membranes.
Acknowledgments
This work was supported by a grant from National Institute of Health (NS056135) to H.A.K. N.S. is a recipient of a predoctoral fellowship from the New Jersey Commission on Spinal Cord Research. We thank Patrice Maurel for helpful discussion and review of this manuscript.
Footnotes
Conflicts of Interest
No potential conflicts of interest to disclose.
References
- 1.Michailov GV, Sereda MW, Brinkmann BG, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 2.Taveggia C, Zanazzi G, Petrylak A, et al. Neuregulin-1 Type III Determines the Ensheathment Fate of Axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Law AJ, Lipska BK, Weickert CS, et al. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5' SNPs associated with the disease. Proc Natl Acad Sci U S A. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes WE, Sliwkowski MX, Akita RW, et al. Identification of heregulin, a specific activator of p185erbB2. Science. 1992;256:1205–1210. doi: 10.1126/science.256.5060.1205. [DOI] [PubMed] [Google Scholar]
- 5.Lu HS, Chang D, Philo JS, et al. Studies on the structure and function of glycosylated and nonglycosylated neu differentiation factors. Similarities and differences of the alpha and beta isoforms. J Biol Chem. 1995;270:4784–4791. doi: 10.1074/jbc.270.9.4784. [DOI] [PubMed] [Google Scholar]
- 6.Marchionni MA, Goodearl AD, Chen MS, et al. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- 7.Ho WH, Armanini MP, Nuijens A, et al. Sensory and motor neuron-derived factor. A novel heregulin variant highly expressed in sensory and motor neurons. J Biol Chem. 1995;270:26722. [PubMed] [Google Scholar]
- 8.Horiuchi K, Zhou HM, Kelly K, et al. Evaluation of the contributions of ADAMs 9, 12, 15, 17, and 19 to heart development and ectodomain shedding of neuregulins beta1 and beta2. Dev Biol. 2005;283:459–471. doi: 10.1016/j.ydbio.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Wang JY, Miller SJ, Falls DL. The N-terminal region of neuregulin isoforms determines the accumulation of cell surface and released neuregulin ectodomain. Journal of Biological Chemistry. 2001;276:2841–2851. doi: 10.1074/jbc.M005700200. [DOI] [PubMed] [Google Scholar]
- 10.Esper RM, Pankonin MS, Loeb JA. Neuregulins: versatile growth and differentiation factors in nervous system development and human disease. Brain Res Rev. 2006;51:161–175. doi: 10.1016/j.brainresrev.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Nave KA, Salzer JL. Axonal regulation of myelination by neuregulin 1. Curr Opin Neurobiol. 2006;16:492–500. doi: 10.1016/j.conb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Velardez MO, Warot X, et al. Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function. J Neurosci. 2006;26:3079–3086. doi: 10.1523/JNEUROSCI.3785-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garratt AN, Voiculescu O, Topilko P, et al. A dual role of erbB2 in myelination and in expansion of the schwann cell precursor pool. J Cell Biol. 2000;148:1035–1046. doi: 10.1083/jcb.148.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanazzi G, Einheber S, Westreich R, et al. Glial growth factor/neuregulin inhibits Schwann cell myelination and induces demyelination. J Cell Biol. 2001;152:1289–1299. doi: 10.1083/jcb.152.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huijbregts RP, Roth KA, Schmidt RE, et al. Hypertrophic neuropathies and malignant peripheral nerve sheath tumors in transgenic mice overexpressing glial growth factor beta3 in myelinating Schwann cells. J Neurosci. 2003;23:7269–7280. doi: 10.1523/JNEUROSCI.23-19-07269.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Syed N, Reddy K, Yang DP, et al. Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J Neurosci. 2010;30:6122–6131. doi: 10.1523/JNEUROSCI.1681-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burkey TH, Hingtgen CM, Vasko MR. Isolation and culture of sensory neurons from the dorsal-root ganglia of embryonic or adult rats. Methods Mol Med. 2004;99:189–202. doi: 10.1385/1-59259-770-X:189. [DOI] [PubMed] [Google Scholar]
- 18.Ogata T, Iijima S, Hoshikawa S, et al. Opposing extracellular signal-regulated kinase and Akt pathways control schwann cell myelination. J Neurosci. 2004;24:6724–6732. doi: 10.1523/JNEUROSCI.5520-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy M, Reddy UR, Pleasure D. Platelet-derived growth factor and regulation of Schwann cell proliferation in vivo. Journal of Neuroscience Research. 1992;31:254–262. doi: 10.1002/jnr.490310206. [DOI] [PubMed] [Google Scholar]
- 20.Eccleston PA, Funa K, Heldin CH. Expression of platelet-derived growth factor (PDGF) and PDGF alpha- and beta-receptors in the peripheral nervous system: an analysis of sciatic nerve and dorsal root ganglia. Dev Biol. 1993;155:459–470. doi: 10.1006/dbio.1993.1044. [DOI] [PubMed] [Google Scholar]
- 21.Oellig C, Pirvola U, Taylor L, et al. Acidic FGF and FGF receptors are specifically expressed in neurons of developing and adult rat dorsal root ganglia. Eur J Neurosci. 1995;7:863–874. doi: 10.1111/j.1460-9568.1995.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 22.Grothe C, Wewetzer K. Fibroblast growth factor and its implications for developing and regenerating neurons. Int J Dev Biol. 1996;40:403–410. [PubMed] [Google Scholar]
- 23.Maurel P, Salzer JL. Axonal regulation of Schwann cell proliferation and survival and the initial events of myelination requires PI 3-kinase activity. Journal of Neuroscience. 2000;20:4635–4645. doi: 10.1523/JNEUROSCI.20-12-04635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brinkmann BG, Agarwal A, Sereda MW, et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59:581–595. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vartanian T, Goodearl A, Viehover A, et al. Axonal neuregulin signals cells of the oligodendrocyte lineage through activation of HER4 and Schwann cells through HER2 and HER3. Journal of Cell Biology. 1997;137:211–220. doi: 10.1083/jcb.137.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baron-Van Evercooren A, Blakemore WF. Remyelination through engrafment. In: Lazzarini R, Griffin J, Lassmann H, Nave KA, Miller R, Trapp B, editors. Myelin biology and disorders. San Diego: Elsevier; 2004. pp. 143–172. [Google Scholar]
- 27.Morrissey TK, Kleitman N, Bunge RP. Human Schwann cells in vitro. II. Myelination of sensory axons following extensive purification and heregulin-induced expansion. J Neurobiol. 1995;28:190–201. doi: 10.1002/neu.480280206. [DOI] [PubMed] [Google Scholar]
- 28.Baron-Van Evercooren A, Gansmuller A, Duhamel E, et al. Repair of a myelin lesion by Schwann cells transplanted in the adult mouse spinal cord. J Neuroimmunol. 1992;40:235–242. doi: 10.1016/0165-5728(92)90139-c. [DOI] [PubMed] [Google Scholar]
- 29.Blakemore WF, Eames RA, Smith KJ, et al. Remyelination in the spinal cord of the cat following intraspinal injections of lysolecithin. J Neurol Sci. 1977;33:31–43. doi: 10.1016/0022-510x(77)90179-4. [DOI] [PubMed] [Google Scholar]
- 30.Duncan ID, Aguayo AJ, Bunge RP, et al. Transplantation of rat Schwann cells grown in tissue culture into the mouse spinal cord. J Neurol Sci. 1981;49:241–252. doi: 10.1016/0022-510x(81)90082-4. [DOI] [PubMed] [Google Scholar]
- 31.Honmou O, Felts PA, Waxman SG, et al. Restoration of normal conduction properties in demyelinated spinal cord axons in the adult rat by transplantation of exogenous Schwann cells. J Neurosci. 1996;16:3199–3208. doi: 10.1523/JNEUROSCI.16-10-03199.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu XM, Zhang SX, Li H, et al. Regrowth of axons into the distal spinal cord through a Schwann-cell-seeded mini-channel implanted into hemisected adult rat spinal cord. Eur J Neurosci. 1999;11:1723–1740. doi: 10.1046/j.1460-9568.1999.00591.x. [DOI] [PubMed] [Google Scholar]
- 33.Pearse DD, Marcillo AE, Oudega M, et al. Transplantation of Schwann cells and olfactory ensheathing glia after spinal cord injury: does pretreatment with methylprednisolone and interleukin-10 enhance recovery? J Neurotrauma. 2004;21:1223–1239. doi: 10.1089/neu.2004.21.1223. [DOI] [PubMed] [Google Scholar]
- 34.Kohama I, Lankford KL, Preiningerova J, et al. Transplantation of cryopreserved adult human Schwann cells enhances axonal conduction in demyelinated spinal cord. J Neurosci. 2001;21:944–950. doi: 10.1523/JNEUROSCI.21-03-00944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lankford KL, Imaizumi T, Honmou O, et al. A quantitative morphometric analysis of rat spinal cord remyelination following transplantation of allogenic Schwann cells. J Comp Neurol. 2002;443:259–274. doi: 10.1002/cne.10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Colognato H. Ffrench-Constant C Contrasting effects of mitogenic growth factors on myelination in neuron-oligodendrocyte cocultures. Glia. 2007;55:537–545. doi: 10.1002/glia.20480. [DOI] [PubMed] [Google Scholar]