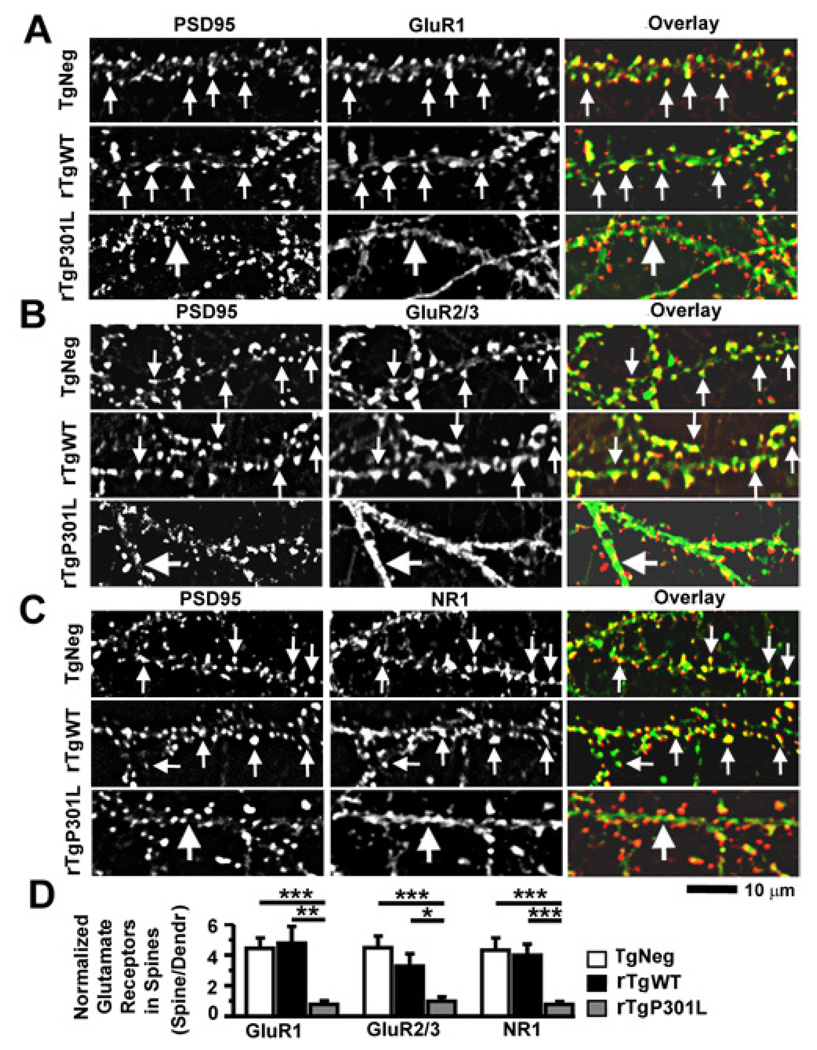
Figure 6. The P301L htau mutation disrupts clustering of GluR1, GluR2/3 and NR1 receptors at dendritic spines in cultured mouse neurons.
(A) The PSD95 protein (left column) and the C-terminus of GluR1 subunits (middle; overlay, right) were co-stained with a mouse monoclonal antibody (red) and rabbit polyclonal antibody (green), respectively. Neurons (21–28 DIV) were cultured from TgNeg, rTgWT and rTgP301L mice (from top to bottom). Note that both surface and intracellular receptors were detected as all primary antibodies were added after the fixation and permeabilization of neurons. (B) Co-staining of PSD95 and GluR2/3 subunits (detected by an antibody that recognizes the C-termini of both GluR2 and GluR3). (C). Co-staining of PSD95 and NR1 (a mandatory subunit for functional NMDARs). (D) Quantification of spine GluR1, GluR2/3 and NR1 immunoreactivity identified by co-localization with PSD95 immunoreactivity, normalized to GluR1, GluR2/3 and NR1 immunoreactivity on adjacent dendritic shafts, respectively. In all images, small arrows denote glutamate receptor clusters that co-localize with PSD95 clusters whereas large arrows denote diffuse staining of glutamate receptors in dendritic shafts. n = 10 neurons per group. ANOVA followed by Fisher’s PLSD post-hoc analysis (D), *p < 0.05, **p < 0.01, ***p < 0.001.