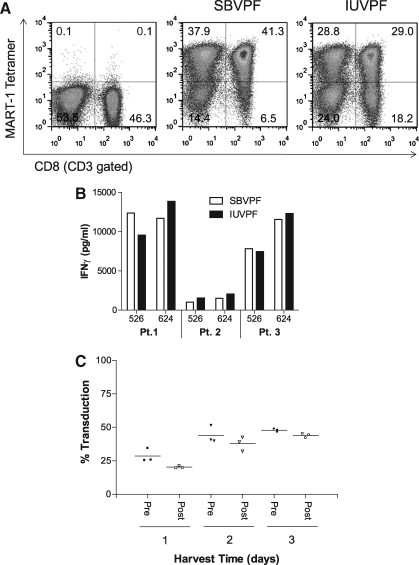
FIG. 1.
Pilot vector production run using expanded surface roller bottles and modified step-filtration. For determination of tetramer-positive cells, PBLs were subjected to fluorescence-activated cell sorting. Analysis of the expression of cell surface markers was carried out using fluorescein isothiocyanate– and allophycocyanin-conjugated antibodies directed against CD3 or CD8 (BD Biosciences). Phycoerythrin-conjugated peptide (MART-127–35)/HLA-A*02 tetramers were purchased from Beckman-Coulter. Vector titers were calculated as follows: (percentage tetramer-positive cells) × (total cells) × (dilution factor)/supernatant volume. (A) Representative fluorescence-activated cell sorting plot of PBLs transduced using the clinical protocol and DMF5 vector produced at both SBVPF and IUVPF. The TCR-transduced PBLs were gated on CD3+/CD8+/tetramer+ cells. (B) Representative data from three patients measuring cytokine release following the incubation of 1 × 105 transduced T lymphocytes with 1 × 105 tumor target cells (HLA-A2+/MART-1+, 526 and 624; HLA-A2-, 888 and 938) for 18 hr. Dilutions of supernatant were then tested for IFNγ by enzyme-linked immunosorbent assay (Thermo Scientific). HLA-A2- tumor lines did not elicit any INFγ secretion (data not shown) (C) Vector recovery following clarification by modified step-filtration and single PBL transductions using the SBVPF DMF5 vector supernatants. Post-clarification SBVPF DMF5 supernatants showed no significant loss of vector titer following modified step-filtration (one-way analysis of variance, p ≥ 0.05). Data presented were from three independent patient transductions.