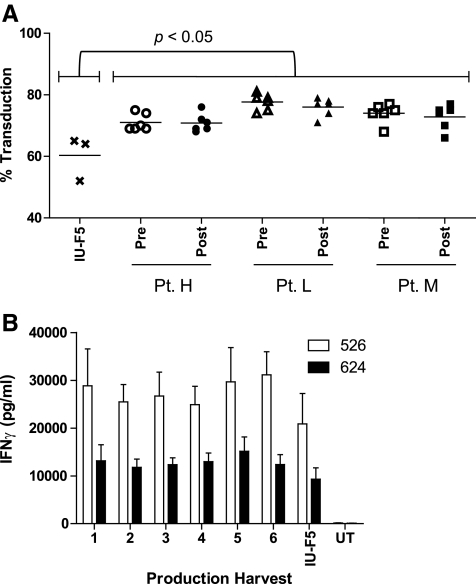
FIG. 2.
Production of cGMP-quality DMF5 gammaretroviral vector. The cGMP MCB encoding the DMF5 TCR [PG13-F5Af2aB (C162D1)] MCB was used to seed 26 1,700-cm2 expanded surface roller bottles for vector production. Samples of vector supernatant were collected pre- and post-filtration following each harvest. (A) Transduction efficiency for each of the six harvests was determined in PBL of three patients following a single transduction as the percentage of CD3+/MART-1 tetramer+ T cells pre- and post-modified step-filtration with no significant reduction in vector titer. As a control, cGMP-quality DMF5 vector supernatant manufactured at IUVPF using step-filtration was used to transduce the same PBLs (one-way analysis of variance, p ≤ 0.05). (B) PBLs from three individual patients were transduced with each of the six vector harvests and tested in duplicate for IFNγ release following co-culture with HLA-A2+/MART-1+ tumor targets. Transduced PBLs were also tested against HLA-A2− tumor targets and showed no specific IFNγ release (data not shown). Errors bars represent ± SEM (p ≥ 0.05).