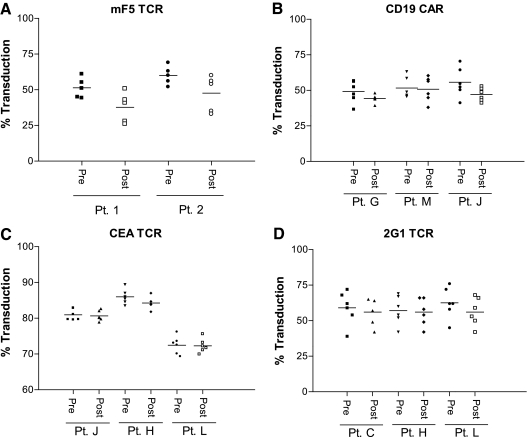
FIG. 3.
Gammaretroviral vector recovery following cGMP manufacturing using modified step-filtration. PG13 MCBs encoding the mF5 MART-1-specific TCR (mF5), CD19-specific CAR (CD19 CAR), CEA TCR, and renal cell carcinoma-specific (2G1) TCR were produced and used to generate cGMP-quality gammaretroviral vector supernatant as described. Transduction efficiency pre- and post-modified step-filtration was assessed for each vector. Each harvest was tested against patient PBLs with the percentage transduction (of the CD3+ T-cell population) shown for each harvest. No significant differences were detected between the pre- and post-filtration vector products when analyzed by one-way analysis of variance (p ≥ 0.05). The horizontal line represents the mean. (A) mF5 transduction efficiency was measured as the percentage of CD3+/MART-1 tetramer+ cells. (B) CD19 CAR detection was determined using a biotin-labeled polyclonal goat anti-mouse F(ab)2 (Jackson Immunoresearch, West Grove, PA) followed by staining with streptavidin-phycoerythrin (BD Pharmingen, San Diego, CA). CD19 CAR transduction efficiency was determined as the percentage of CD3+/CD19 CAR+ cells. (C) CEA TCR transduction efficiency was measured as the percentage of CD3+/murine β chain constant region (eBiosciences, San Diego)-positive cells. (D) 2G1 TCR expression was measured as the percentage of CD3+/anti-Vβ2 antibody-positive cells (Beckman Coulter).