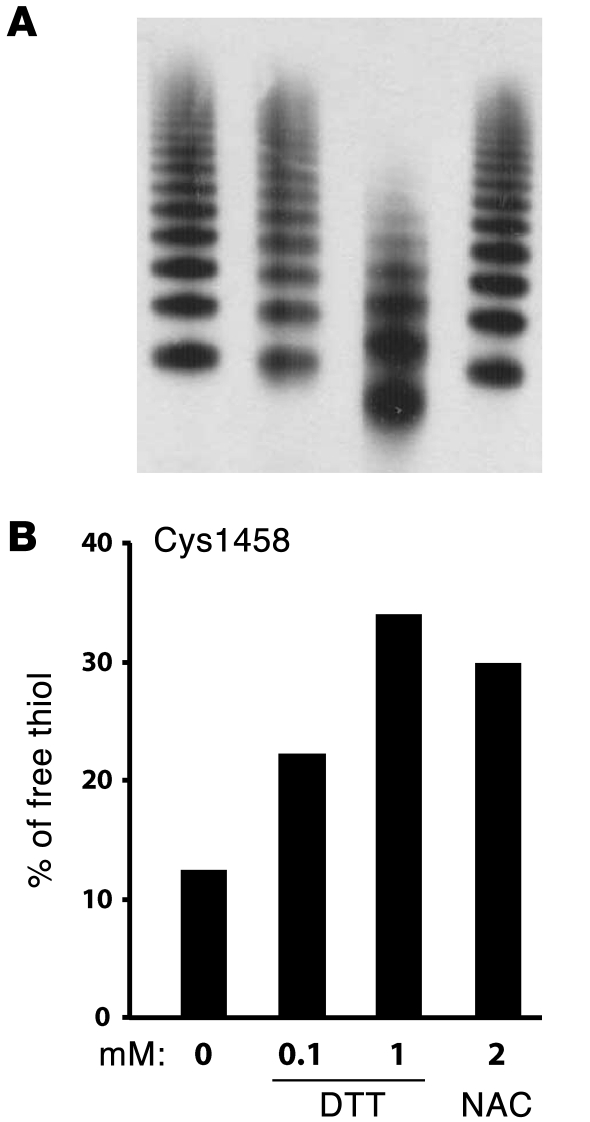
Figure 3. DTT and NAC reduced the Cys1272–Cys1458 disulfide bond in the vWF A1 domain under shear.
Purified plasma vWF was sheared on a cone-and-plate viscometer at 37°C, 10,000 s–1 for 15 minutes in the presence of 25 mM HEPES buffer (0 mM), DTT, or NAC. The samples were then mixed immediately with NEM to prevent further disulfide bond shuffling. The percentage of Cys1458 in the vWF A1 domain existing as a free thiol was quantified by mass spectrometry. (A) vWF multimers were examined by SDS-agarose electrophoresis and immunoblotting with a polyclonal vWF antibody. (B) The percentage of Cys1458 existing in the free thiol form increased in the presence of DTT or NAC.