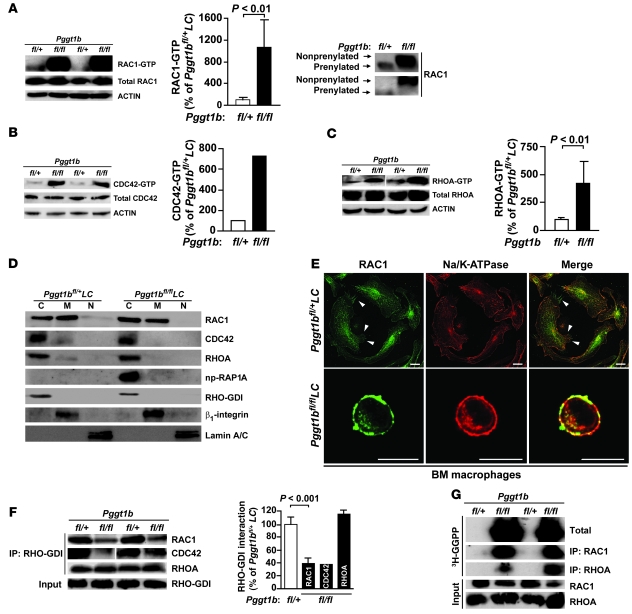
Figure 3. Knockout of GGTase-I results in accumulation of GTP-bound RHO family proteins and has little effect on the subcellular localization of RAC1.
(A–C) Western blots show levels of GTP-bound and total RAC1 (A), CDC42 (B), and RHOA (C) in lysates from BM macrophages. Actin was used as a loading control. Corresponding graphs show the amount of GTP-bound RAC1 (n = 7), CDC42 (n = 2), and RHOA (n = 4) determined by densitometry. Reduced electrophoretic mobility of affinity-purified RAC1 in lysates from Pggt1bfl/flLC macrophages (run on 12% Protean gels) is also shown in A. (D) Western blots showing the distribution of RAC1, CDC42, RHOA, and np-RAP1A in the cytosol (C), membrane (M), and nuclear (N) fractions of BM macrophages. RHO-GDI, β1-integrin, and lamin A/C were used as markers for cytosol, membrane, and nuclear fractions, respectively. (E) Confocal micrographs showing immunohistochemical staining of BM macrophages with antibodies against RAC1 and Na/K-ATPase. Arrowheads indicate RAC1 at filopodia at the plasma membrane. Scale bars: 10 μm. (F) Immunoprecipitation of RHO-GDI in lysates of BM macrophages followed by Western blot for RAC1, CDC42, and RHOA. Levels of RHO-GDI–bound RAC1 (n = 4), CDC42 (n = 2), and RHOA (n = 4), as determined by densitometry, are also shown. Lanes were run on the same gel but were noncontiguous (white line). (G) Accumulation of unprocessed GGTase-I substrates susceptible to in vitro prenylation by 3H-GGPP and recombinant GGTase-I. The experiment was repeated twice with similar results.