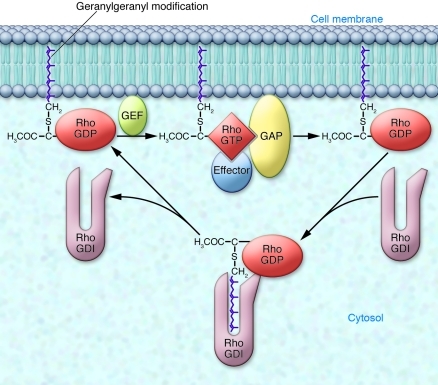
Figure 1. Regulation of geranylgeranylated Rho GTPases.
Inactive Rho proteins are GDP bound and sequestered in the cytosol by binding to their chaperone RhoGDI, which shields the geranylgeranyl group from the aqueous environment. The activation cycle begins when Rho proteins translocate using their geranylgeranyl lipid moieties to cellular membranes, where they encounter GEFs that catalyze GTP/GDP exchange. GTP-bound Rho proteins engage effectors and are acted on by GAPs that facilitate GTP hydrolysis, returning the Rho proteins to their inactive state that can be extracted from the membrane by free RhoGDI. Central to each step of regulation is the geranylgeranyl lipid. However, in light of the findings of Khan et al., who show that macrophages can function without geranylgeranylation of Rho proteins (3), the role of the lipid modification may have to be reconsidered.