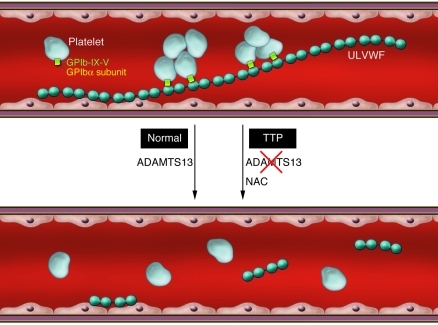
Figure 1. Regulation of vWF-dependent thrombosis under shear conditions.
vWF is normally expressed as ultralarge, prothrombotic multimers on activated endothelial cells. These ultralarge multimers are prothrombotic in that they support the initial adhesion of circulating platelets via an interaction with the GPIbα subunit of the platelet receptor GPIb-IX-V, leading to microthrombi formation. In healthy individuals, ADAMTS13 cleaves within the vWF-A2 domain, releasing smaller vWF multimers into the circulation that no longer spontaneously bind platelets. In patients with TTP, ADAMTS13 activity is deficient, resulting in vascular microthrombi. In their study in this issue of the JCI, Chen et al. (2) show that the drug NAC can also decrease vWF multimer size by reducing intersubunit disulfide bonds to form smaller vWF multimers, following initial reduction of the intrasubunit disulfide (C1272–C1458) of the A1 domain.