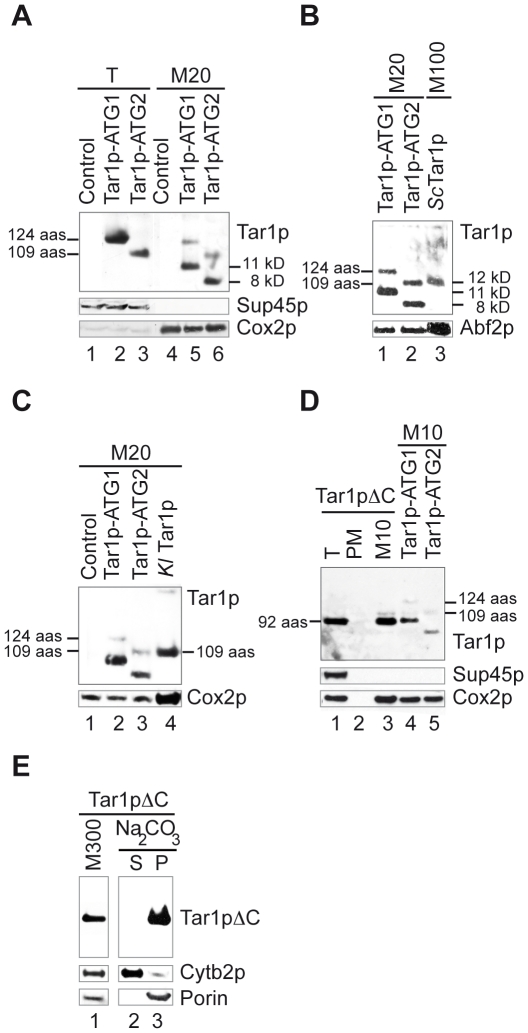
Figure 4. Sc Tar1p polypeptide truncated at its N-terminus or C-terminus co-fractionates with mitochondria.
(A) Total cell extracts (T, 5 µg) and mitochondrial extracts (M20, 20 µg) from W303 strain expressing an empty plasmid (control) or plasmid-version of Tar1p (Tar1p-ATG1, 124 aas) or of N-terminal truncated Tar1p (Tar1p-ATG2, 109 aas). The amino-acids length of plasmid-derived polypeptides is indicated. The apparent molecular weight of smaller immunoreactive species detected in mitochondrial extracts is indicated. (B, C) Compared electrophoretic mobility between endogenous Sc Tar1p, endogenous Kl Tar1p and plasmid-derived Tar1p-ATG1 and Tar1p-ATG2 in mitochondrial extracts. Mitochondrial extracts are the same as in (A) and from S. cerevisiae and K. lactis wild type strains (see Figure 2). (D) Total cell extracts (T, 10 µg), postmitochondrial supernatant (PM, 10 µg) and mitochondria (M10, 10 µg) from W303 strain expressing a plasmid-derived C-terminal truncated Tar1p (Tar1pΔC, 92 aas). Mitochondria (M10, 10 µg) from W303 strain expressing Tar1p-ATG1 or Tar1p-ATG2 were loaded in parallel to compare the apparent molecular weight of the different immnuoreactive species. Lane 3, the thin band detected above the Tar1pΔC signal may correspond to endogenous Sc Tar1p. (E) Mitochondria (M300, 300 µg) from W303 strain expressing Tar1pΔC were incubated with Na2CO3 and separated into soluble supernatant (S) and membrane pellet (P) fractions. The different Tar1p polypeptides were revealed using the specific anti-Tar1p antibody.