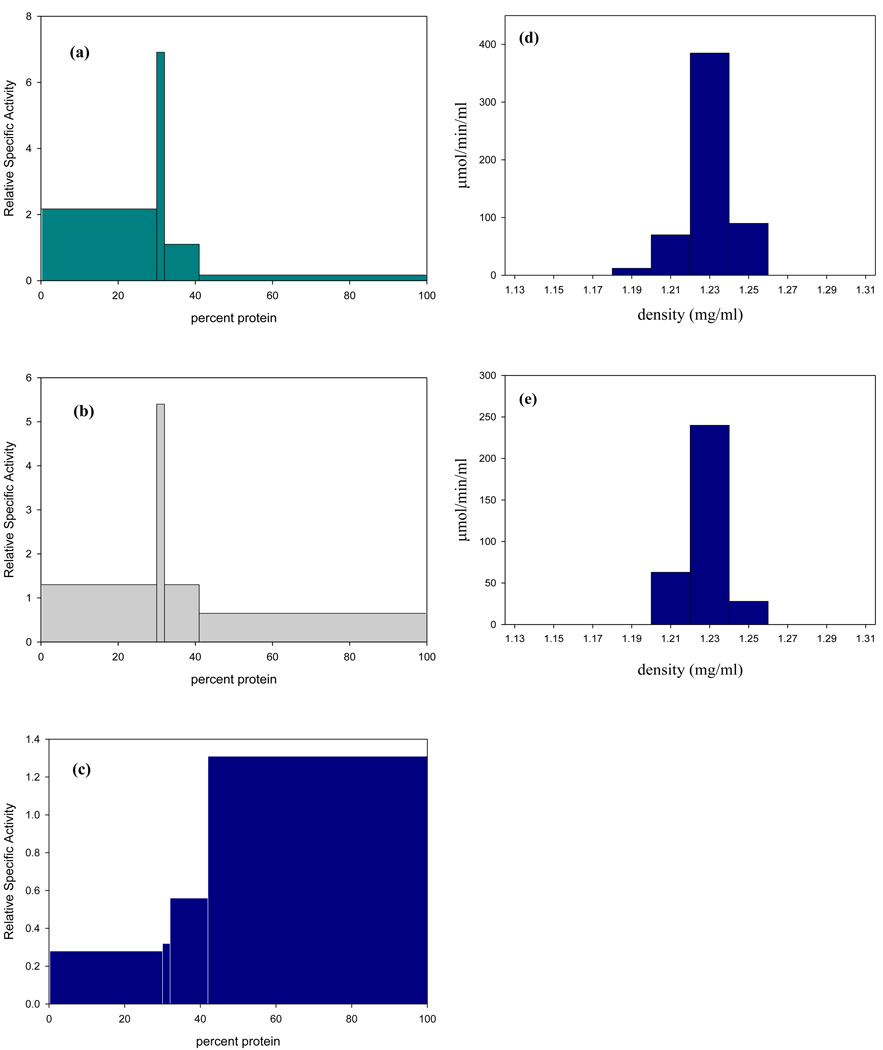
Figure 2. Subcellular localization of ADH enzymes in T. vaginalis isolate (SS-22).
Distribution of enzymes in fractions obtained by differential centrifugation of a cell-free extract of T. vaginalis SS-22 in 225 mM sucrose-10 mM Tris pH 7.4, containing 1 mM calcium chloride and 1 mM magnesium chloride. Relative specific activities (the ratios of specific activities in fractions to those in the cell-free extract) were plotted against cumulative percentage protein recovered in each fraction. The centrifugal force increases from left to right such that the fractions represent the nuclei (400 × g for 10 min), hydrogenosomes (2,200 × g for 10 min), lysosomes (28,000 × g for 30 min), and the non-sedimentable fraction (>28,000 × g for 30 min). Percent recovery for each enzyme after subcellular fractionation was: (a) ADI, 87%; (b) MDH, 93%; (c) cOCT, 98%. Hydrogenosomes obtained by differential centrifugation were further purified using 50% (w/v) percoll gradients containing 225 mM sucrose-10 mM Tris pH 7.4, 1 mM calcium chloride and 1 mM magnesium chloride. One ml of the hydrogenosome-enriched fraction was centrifuged at 46,000 g for 45 min at 4°C. Fractions were assayed for ADI and MDH activity as described; (d) ADI had a single peak of activity at a density of 1.23 g/ml, percent recovery was 84%; (e) The hydrogenosomal marker enzyme MDH had a single peak of activity at the same isopycnic density observed for ADI, percent recovery was 92%.