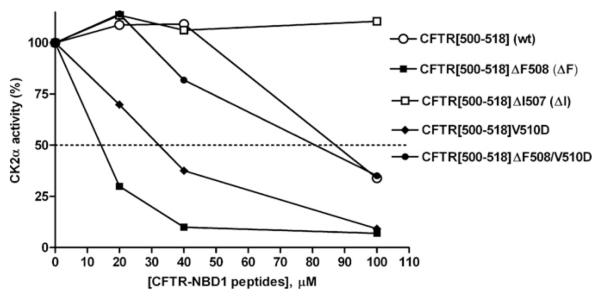
Figure 1. Sequence-determined variability of inhibition of CK2α by short CFTR peptides straddling the Phe508 region of wild-type or mutant CFTR.
The percentage change in CK2α activity (ordinate, relative to no-peptide control as 100%) was determined against the specific synthetic peptide RRRADDSDDDDD as phosphorylatable substrate. A bi-directional effect on CK2α occurs between Phe508 and negative charge compared with hydrophobicity at amino acid 510. For this and subsequent Figures, CK2α activity was determined as described in the Experimental section and all data points are calculated from the mean of at least three independent experiments with the S.E.M. never exceeding 15% (not shown for clarity).