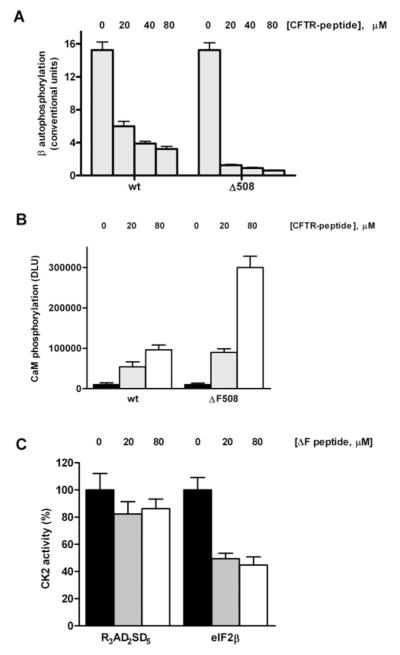
Figure 6. Variable effects of the interaction of CK2 holoenzyme with the wild-type and ΔF508 CFTR-derived peptides.
Autophosphorylation of the CK2 β-subunit and assays on holoenzyme activity towards substrates (peptide and protein) were performed as described in the Experimental section. (A) Inhibition of the CK2 β-subunit autophosphorylation by increasing concentrations of either wild-type or ΔF508 CFTR peptides. (B) Stimulation of the phosphorylation of calmodulin induced by increasing concentrations of either wild-type or ΔF508 CFTR peptides (contrast with the Vmax decline in Figure 3A). Note the enhanced relative potency of the Phe508-deleted peptide towards calmodulin phosphorylation (compare open bars) when holoenzyme is present against its contrasting effects on CK2α alone. However, in (C), the very same peptide has inhibitory actions towards the phosphorylation of the eIF2β-derived peptide as the concentrations of this CFTRΔF508 peptide rise. Note no effect when eIF2β-derived peptide (class III substrate) is replaced by another peptide (class I substrate) showing selectivity of action towards different substrates.