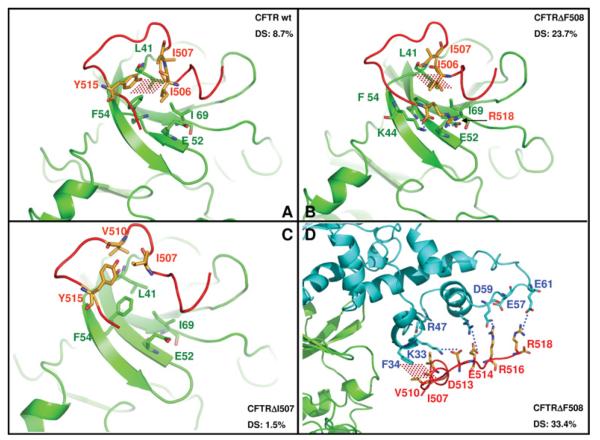
Figure 8. Molecular docking of wild-type and mutated CFTR peptides by CK2α and by CK2 holoenzyme.
In (A), (B) and (C), the main molecular contacts between CK2α (green), CFTR peptides (red) and CK2β (blue; D only) are shown as complexes between CK2 α-subunit and CFTR peptides (wild-type, ΔF508 or ΔI507). Using Glu52/Ile69 (green) as a CK2α fixed reference points, note the different contacts for Phe54 and Leu41 (both green) against differently folded CFTR peptides in (A), (B) and (C). In (D), the interactions between the β-subunit of the CK2 holoenzyme and the CFTRΔF508 peptide are highlighted. Dotted red areas denote hydrophobic interactions, blue dotted lines indicate salt bridges. The DS parameter for each complex, calculated as described in the Experimental section and discussed in the text, is also shown.