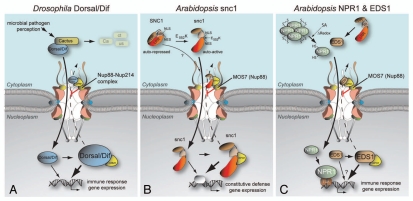
Figure 1.
Nup88 promotes nuclear retention of animal and plant immune regulators. (A) The Drosophila homolog of vertebrate Nup88, Members only (Mbo), is required for nuclear accumulation of the Rel-type transcription factors Dorsal and Dif. Toll receptor signaling upon microbial infection releases the NFκB homologs Dorsal/Dif from the inhibitory IκB homolog Cactus, allowing Dorsal/Dif nuclear translocation. During this immune response, Mbo/Nup88 attenuates nuclear export of Dorsal/Dif by sequestering the Dorsal/Dif-loaded export receptor CRM1 at the nuclear rim. This attenuation in nuclear export results in nuclear accumulation of Dorsal/Dif and efficient defense gene expression. In contrast, mbo mutant animals show enhanced nuclear export of Dorsal/Dif and fail to activate an immune response (Roth et al. 2003; Uv et al. 2000; Xylourgidis et al. 2006).14,13,15 (B) In Arabidopsis, an E552K change in SNC1 renders this TIR-NB-LRR immune receptor constitutively active without a pathogen stimulus.3 Autoactive snc1 localizes to the cytoplasm and the nucleus and possibly shuttles between the two compartments via NLS/Importin-mediated nuclear import and NES/XPO1-mediated nuclear export. Autoimmunity of snc1 requires functional MOS7 (Nup88) since a partial loss-of-function mutation in mos7-1 results in XPO1-mediated nuclear leakage of snc1 and suppression of all known snc1 auto-immune phenotypes. This is consistent with MOS7 promoting nuclear retention and accumulation of autoactive snc1 as a critical process in the constitutive activation of immune responses.17 (C) MOS7 (Nup88) is also required for proper nuclear accumulation of the plant-specific defense regulators NPR1 and EDS1. NPR1 translocates to nuclei of uninduced tissues at a low rate.24 Induction by the plant defense hormone SA and subsequent changes in the cellular redox state promote NPR1 monomerization via thioredoxin-mediated reduction of intermolecular disulfide bonds and nuclear accumulation of NPR1 monomers, a process required for efficient expression of NPR1-regulated defense genes and induction of systemic immunity.19 EDS1 is a key component of immunity triggered by snc1 and other intracellular TIR-NB-LRR immune receptors.25,5 Nucleocytoplasmic EDS1 forms several distinct defense regulatory complexes in the cytoplasm and the nucleus (not shown) and is capable of XPO1-mediated nuclear export.26,27 Altered nuclear translocation rates of NPR1 and EDS1 in mos7-1 might affect NPR1 and EDS1 protein stability that becomes sensed and equilibrated across the NE over time.