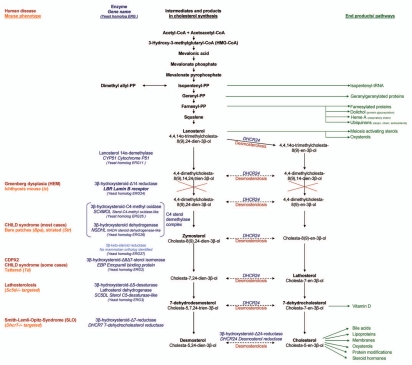
Figure 7.
Cholesterol synthesis pathway, associated diseases and putative pathogenic mechanisms (reviewed in refs. 26, 49 and 50). Blockade of the C14 sterol step might lead to an accumulation of precursors with subsequent up or downregulation. This accumulation could affect important pathways such as farnesylation, heme and ubiquinone synthesis, or even the first hydroxyl methyl glutaryl CoA reduction step. Equally or more likely might be a deficiency in downstream products. LBR catalyzes an early step in post-squalene cholesterol synthesis. Failure in this step might subsequently result in different amounts or composition of derivates from intermediate steps, such as meiosis activating sterols, oxysterols, vitamin D, and finally of the end-product cholesterol and its derivates bile acids and steroid hormones.26 Cholesterol is a major component of membranes and lipid rafts and is produced in significant amounts by the fetus itself.51 Insufficient amounts and altered membrane composition could impair fetal development. Greenberg dysplasia could even feature a modified hedgehog pathway, as a result of cholesterol modification. Hedgehog proteins are modified by cholesterol.52 Altered hedgehog signaling was shown in other diseases of the post-squalene pathway35 and mutations in genes of the hedgehog pathway cause a number of skeletal defects. These defects include brachydactyly and polydactyly that are also seen in Greenberg dysplasia. Impaired vitamin D metabolism might be another potential effector in Greenberg dysplasia since LBR affects a step upstream of the vitamin D precursor 7-dehydrocholesterol. Vitamin D is essential for bone development. Vitamin D is produced in significant amounts in the placenta and the fetus itself. Though to our knowledge, whether or not there is de novo synthesis of vitamin D in the fetus is not entirely clear; however, at least locally such synthesis might be possible. There are overlapping pathophysiologic and histologic findings in Greenberg dysplasia, rickets in children, and osteomalacia in adults. Skeletal mineralization depends on the presence of sufficient amounts of calcium and phosphate at the sites of mineralization. Furthermore, chondrocytes, osteoblasts and collagen matrix must position and function properly. Mineralization occurs in chondrocytes. If osteoblasts produce more matrix than the chondrocytes can mineralize, rickets or osteomalacia can develop. Similar mechanisms could be operative in a very early stage of skeletal development in Greenberg dysplasia. The imbalance could either be due to absence or mal-position of calcium by vitamin D deficiency or by abnormal chondrocyte function. In addition, rickets show inadequate mineralization of the chondrocyte matrix in the growth plates. In both rickets and Greenberg dysplasia there is a disorganization or complete failure of chondrocytes to form chondrocyte columns. Epiphyses are stippled, growth of long bones is impaired in both conditions. Osteomalacia is also seen in neurofibromatosis and as a complication of anticonvulsive therapy.53