Figure 1.
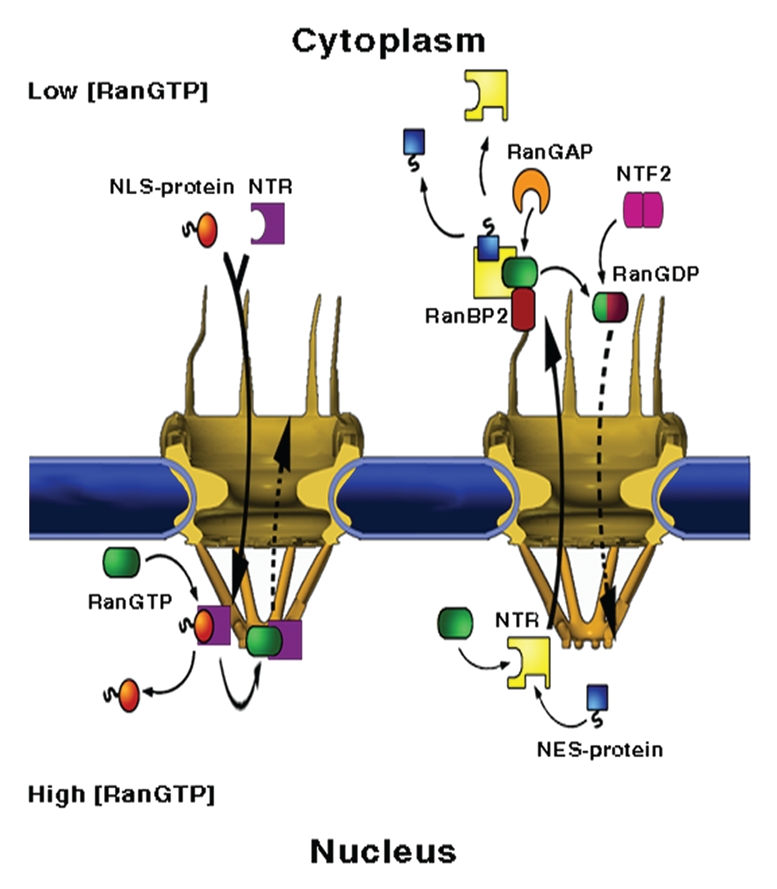
NPC structure and mediated-transport cycles. NPCs fuse the inner and outer membranes of the nuclear envelope (blue), forming aqueous channels that communicate between the nucleus and the cytoplasm. The vertebrate NPC measures about 120 × 90 nm and is made up of ∼30 different proteins, called nucleoporins or Nups, most of which are present in multiples of eight. Associated with the core scaffold of the assembly are eight, ∼50 nm long filaments that protrude towards the cytoplasm and a massive, fish trap-like structure, termed the nuclear basket, which extends about 50 nm into the nucleoplasm. (Left part) Nuclear import. A protein carrying a nuclear localisation signal binds to an import transport receptor in the cytoplasm. Upon reaching the nucleoplasmic face of the pore, binding of RanGTP to the transport receptor frees the latter from FG-repeats in the pore and dissociates the complex. (Right part) Nuclear export. A ternary export complex is formed between a nuclear export receptor, a nuclear export signal-bearing cargo and RanGTP, which typically increases the affinity of export receptors to their cargo. The complex traffics to the cytoplasmic face of the NPC where it is disassembled and the Ran-bound GTP is concomitantly hydrolysed in a process requiring one of two Ran-binding proteins, called RanBP1 and RanBP2 and Ran's GTPase-activating protein RanGAP1. Due to its small size, Ran can, in principle, cross the NPC by passive diffusion. However, to maintain a steep RanGTP gradient across the NE, the transport of GDP-loaded Ran back to the nucleus, where recharging with GTP takes place, is facilitated by a dedicated import receptor called NTF2.
