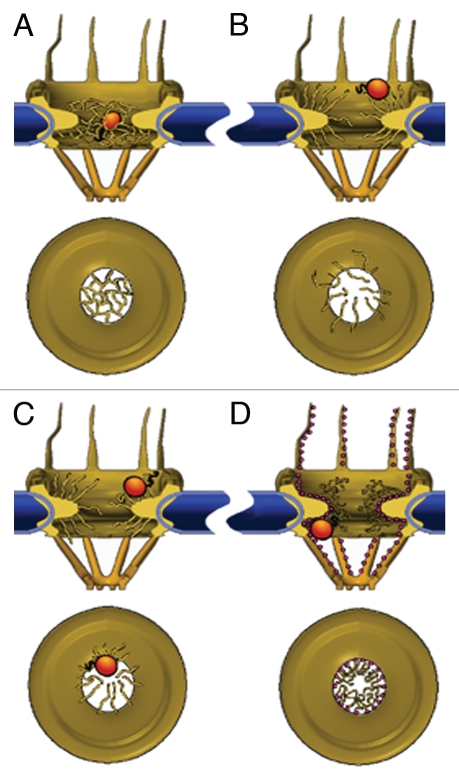
Figure 2.
Models of the NPC permeability barrier. (A) Self-interacting FG-repeats form a meshwork within the central pore channel; only molecules that are smaller than the mesh size can go through unassisted. NTRs partition into the meshwork by replacing internal FG-FG contacts with interactions with their hydrophobic patches, virtually ‘dissolving’ into the polymer gel. (B) Unlinked FG-repeats at the edges of the NPCs form polymer brushes that exclude macromolecules through their thermal motion. NTRs permeate this barrier by binding to the FG-repeats, thereby increasing their probability of entry. (C) NTR binding induces collapse of FG-domains, which is reversed by dissociation of the receptor. Transport is afforded by repeated collapse and re-extension events. (D) FG-repeats (shown here as small red spheres) collapse to form a continuous layer extending from the cytoplasmic filaments, through the interior of the central pore channel, to the nuclear filaments. NTRs glide over this surface in a 2D random-walk fashion whereas small molecules pass through an aqueous tube located at the centre of the conductive channel. The space between this tube and the FG-layer in the channel is assumed to be occupied by a loose network of hydrophilic flexible polypeptides, possibly the hydrophilic stretches that connect between the FG-repeats.