Figure 4.
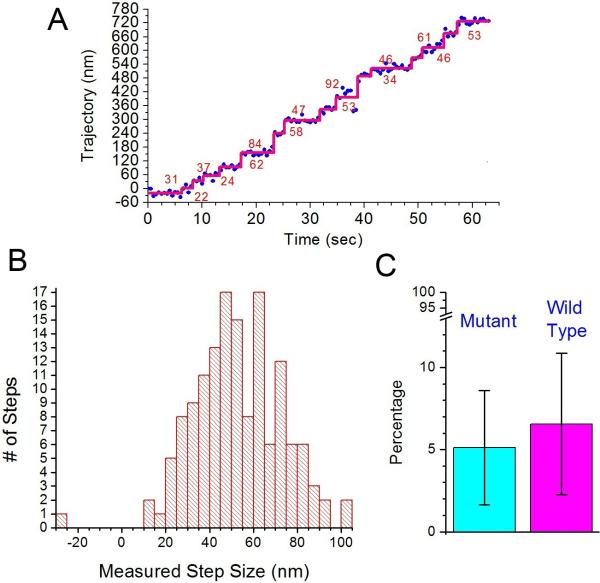
Insensitivity of processivity to mutations of hydrophobic residues in the medial tail. (A) Example trace of the mutant 940 construct (see text). The graph plots the position of the fluorescent labeled CY3 on one of the IQ-CaMs along actin filament versus time. Calculated step sizes are denoted by numbers in the graph. (B) Measured step size histogram for the mutant 940 construct. The average forward step size is 53.3±19.1 nm (N=143) and the average backward step size is -26.3 nm (N=1). (C) Comparison of the percentage of properly dimerized myosin VI 940 for WT and mutant-type constructs. A fraction of 5.1±3.5 % and 6.6±4.3 % (average ± s.d.) of myosin VI was seen to be processive for mutant- and WT constructs, respectively. Percentages were calculated from nine separate measurements; a total of 1007 and 995 myosins was counted for mutant- and WT, respectively. Ionic strength of buffer was 52 mM for both cases.