Table 1. Synthesis of α-Allyl Imidates.
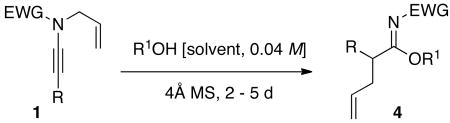 | ||||
|---|---|---|---|---|
| Entry | Alcohol | Temp/Time | Imidate | Yield [%]a |
| 1 | MeOH | 75 °C/2 d | 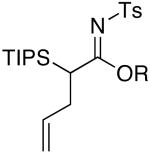 |
4a: R = Me [81%] |
| 2 | EtOH | 75 °C/2 d | 4b: R = Et [43%] | |
| 3 | i-PrOH | 90 °C/5 d | 4c: R = i-Pr [39%] | |
| 4 | c-pentanol | 110 °C/5 d | 4d: R = c-pent [45%] | |
| 5 | EtOH | 85 °C/3 d | 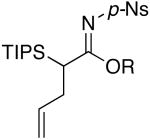 |
4e: R = Et [76%] |
| 6 | i-PrOH | 90 °C/4 d | 4f: R = i-Pr [76%] | |
| 7 | c-pentanol | 115 °C/4 d | 4g: R = c-pent [71%] | |
| 8 | MeOH | 75 °C/2 d | 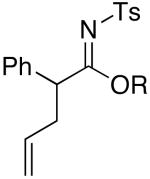 |
4h: R = Me [95%]c |
| 9 | EtOH | 75 °C/2 d | 4i: R = Et [75%] | |
| 10 | i-PrOH | 75 °C/5 d | 4j: R = i-Pr [47%]d | |
| 11 | EtOH | 75 °C/2 d |
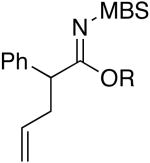 b b
|
4k: R = Et [82%] |
| 12 | c-pentanol | 75 °C/2 d | 4l: R = c-pent [38%] | |
Isolated Yields.
MBS = p-methoxybenzenesulfonyl.
No nitrile observed in crude 1H NMR.
Est. nitrile yield by crude 1H NMR = 20%.
