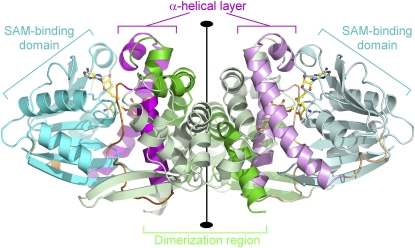
Figure 3.
Structure of a Homodimer of the Lp OMT1/SAH/Sinapaldehyde Complex.
The homodimer is viewed perpendicular to the twofold rotational axis (vertical) that relates the two monomers. For each of the monomers, the polypeptide chain backbone is color coded by structural domain: dimerization domain (residues 1 to 158), green; SAM/SAH binding domain (residues 199 to 295 and 328 to 360), cyan; α-helical layer involved in phenolic substrate binding (residues 159 to 192 and 304 to 323), magenta; and hinge segments (residues 193 to 198, 296 to 303, and 322 to 327), orange. (One of the monomers is shown with slightly paler shading.) S-adenosyl-homocysteine and sinapaldehyde are shown in ball-and-stick representation and colored according to atom type (carbon, yellow; nitrogen, blue; oxygen, red; and sulfur, gold).