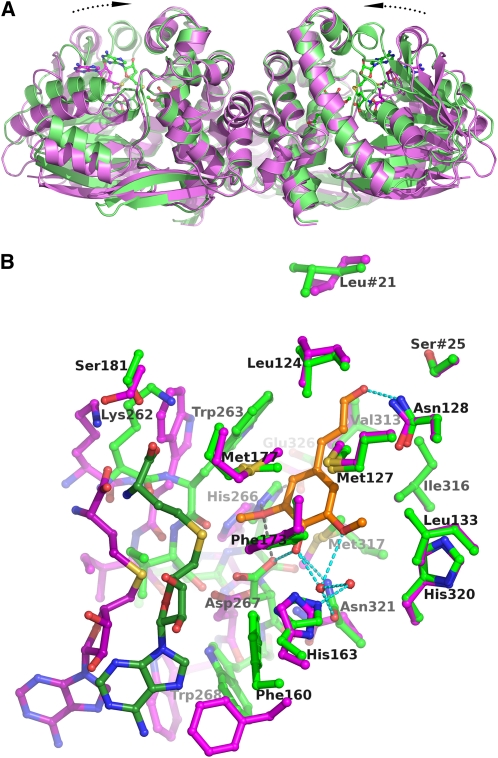
Figure 4.
Comparison of the Open and Closed Conformational States of Lp OMT1.
(A) Comparison of the holoenzyme and holoenzyme/sinapaldehyde forms of the OMT1 homodimer. The structural superposition is based solely on the dimerization domains of the two forms of Lp OMT1. The OMT1 holoenzyme (magenta, with carbon atoms of SAH drawn in darker magenta) exists in an open conformational state, whereas the holoenzyme/sinapaldehyde form (green, with carbon atoms of SAH and sinapaldehyde drawn in darker green) is significantly more closed. The closed conformation of the holoenzyme/sinapaldehyde form arises from an inward rotation (by ~17°) of each SAM binding domain. The orientation of view is identical to that in Figure 3.
(B) Comparison of the active sites in the open and closed conformational states of OMT1. The structures of the OMT1 holoenzyme (open, magenta) and holoenzyme/sinapaldehyde (closed, green) are colored as in (A), except that the carbon atoms of sinapaldehyde are colored orange. With the closed conformational state, the substantial inward shift of the SAM binding domain is evident at the left of the figure (only SAH and residues 262 to 268 and Glu-326, which include the key catalytic residues, are shown). Hydrogen bond interactions involving the sinapaldehyde ligand are shown as dashed lines and are colored as in Figure 5A. The most significant side chain conformational differences between the two forms of OMT1 occur at Phe-160, Ser-181, Trp-263, Met-317, and Asn-321.