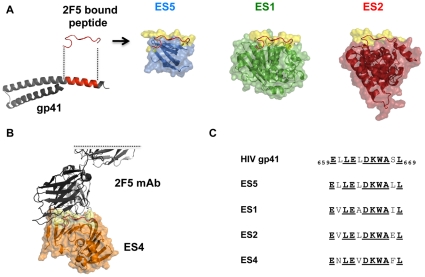
Figure 1. The 2F5 epitope scaffold (ES) fusion proteins.
(A) Left, in red the 2F5 gp41 epitope region is shown both in the post-fusogenic helical form (PDB 3K9A) and in the 2F5-bound conformation (PDB 1TJI). The gp41 membrane proximal external region (MPER) including the 2F5 epitope adopts most frequently an alpha-helical conformation, however, it forms an extended ß-turn loop conformation when bound to the 2F5 antibody, as described in [48]. Structural models (pymol) of the ES proteins used as immunogens ES5 (blue), ES1 (green) and ES2 (red), respectively. Their molecular surfaces are rendered translucent to display the underlying secondary structure. Superimposed (in red) is the 2F5 antibody-bound peptide conformation. The conserved 2F5 epitope graft molecular surface is shown in yellow. (B) Partial structure of the 2F5 antibody Fab (gray) docked to the model of ES4 (orange). ES4 was used as an antigenic probe to measure epitope-specific responses to the conformationally constrained 2F5 epitope and was not used as an immunogen. (C) Alignment of the gp41 2F5 epitope and the ES graft sequences; the 2F5 antibody contact residues defined in the 2F5 antibody-peptide structure are emboldened and underlined.