Abstract
We saw two cases of gastrointestinal stroma tumour (GIST) in morbid obese patients 6 months ago. They were diagnosed with endoscopic ultrasonography. We used laparoscopic sleeve gastrectomy (LSG), a new bariatric surgery, in order to treat morbid obesity and GISTs at the same time. After the operation, the GISTs were removed successfully. The body weights and fasting glucose levels decreased significantly. As a result, LSG is a good and simple method in treating GISTs in morbid obese patients.
BACKGROUND
Morbid obesity is now threatening populations in developing countries, particularly those of South America, Asia and Africa.1
Gastrointestinal stroma tumours (GISTs) are uncommon neoplasms found most often in the stomach (60–70%) followed by the small intestine (20–25%).2 Approximately 70% of patients with GISTs are symptomatic, 20% are asymptomatic and 10% are detected at autopsy.3 Surgical resection of the primary tumour is the treatment of choice for patients with GISTs. GIST is the subject of controversy not only because of its origin but also due to its differentiation, classification and prognosis.
GIST combined with morbid obesity is rare and difficult to diagnose in clinic. At present we are not sure the exact relation between GISTs and morbid obesity. Most patients have no symptoms in the early stage. Some patients are diagnosed other reasons, such as a health test or other diseases. We met two patients recently. They intended to receive bariatric surgery for morbid obesity without any symptoms in the alimentary tract. They were diagnosed with endoscopic ultrasonography.
The patients had a strong desire to finish bariatric surgery in order to control their body weights. We gave up the bypass or lap band surgery because we were not sure if the GISTs would be stimulated by the internal environment changes. Laparoscopic sleeve gastrectomy (LSG) was the best way to remove the tumour and at the same time their body weights could be controlled.
CASE PRESENTATION
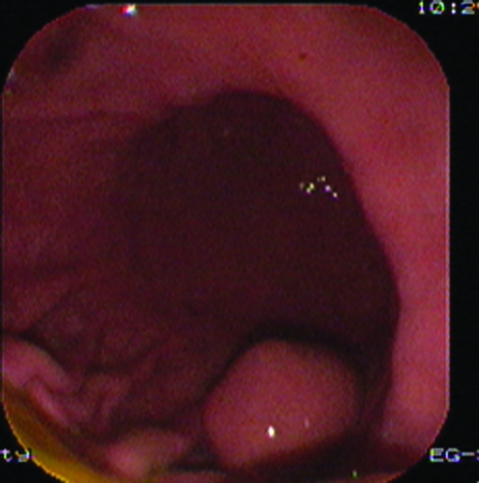
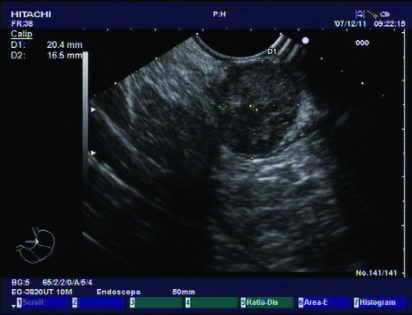
Case 1 was a 26-year-old married woman, a teacher, without children. Nobody in her family was ever diagnosed with GIST or morbid obesity before. Her body weight was 110 kg. Height was 165 cm. Her body mass index (BMI) before the operation was 40.3 kg/m2. Her blood pressure was 145/89 mmHg. Fasting glucose was 6.68 mmol/L. Preoperative endoscopic ultrasonography examination (PENTAX EG-2940 endoscope/PENTAX EPM-3500, PENTAX EG-3830UT linear-array echo endoscope/HITACHI EUB-6500, Tokyo, Japan),showed a submucosal tumour about 2 cm in diameter, which was located in the larger curve of the stomach (figs 1 and 2).
Figure 1.
Findings from endoscopy.
Figure 2.
Findings from endoscopic ultrasonography.
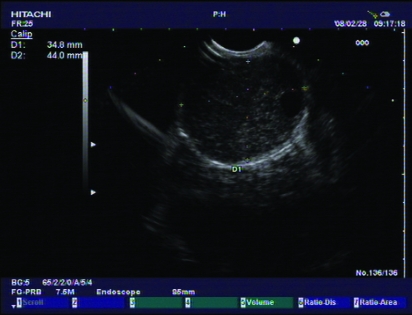
Case 2 was a 43-year-old woman, occupation cook, married with one child. She also had no subsequent family medical history. Her body weight was 118 kg. Her height was 162 cm. Her BMI was 45 kg/m2. Her blood pressure was 200/110 mmHg. Fasting glucose was 7.1 mmol/L. She was also diagnosed with GISTs (3.5×4.4 cm) with ultrasonography examination (figs 3 and 4).
Figure 3.
Endoscopic findings.
Figure 4.
Endoscopic ultrasonography findings.
DIFFERENTIAL DIAGNOSIS
It had to be differentiated with gastric adenocarcinoma from pathological diagnosis.
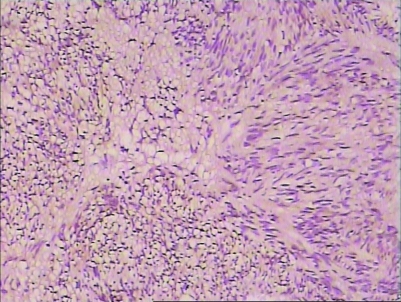
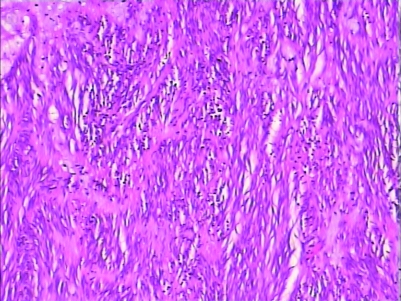
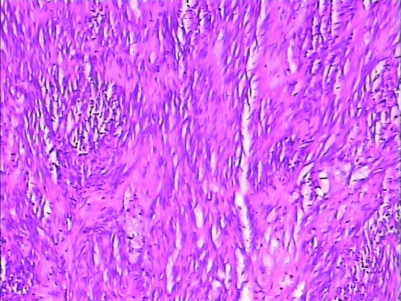
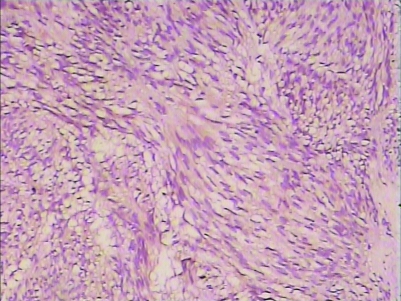
H&E staining and immunohistochemistry staining could be used to diagnose (figs 5–8).
Figure 5.
In operation, the gastric tube has been created.
Figure 8.
CD117 staining of GIST (10*25).
Figure 6.
H&E staining of GIST (10*25).
Figure 7.
H&E staining of GIST (10*25).
Figure 9.
CD117 staining of GIST (10*25).
TREATMENT
Under general anaesthesia, the patients received aeroperitoneum (15 mmHg). A camera port (12 mm, periumbilicus) was built up to insert laparoscope. Other four ports (one 12 mm, one 10 cm and two 5 mm) were used for operation. First, it was proven there was no metastasis in abdominal cavity. Then larger curvature of the stomach was disclosed. The location of the tumour was confirmed by an intraoperative gastroscopy. A gastric tube was created using repeated firing with linear staplers (60 mm, Ethicon Endosurgery, Cincinnati, Ohio, USA) from the distal antrum (6 cm from the pylorus) to the Hiss angle, with complete removal of the greater curvature and fundus (fig 5). At the same time, GISTs in the larger curve were also removed. The port of 12 mm was enlarged to take samples out.
OUTCOME AND FOLLOW-UP
The operation continued for 97 mins and 103 mins for each patient, respectively. Bleeding for each patient was about 200 ml and 350 ml, respectively. Three days later, the patients recovered completely. After 6 months and 3 months follow-up, no complications occurred in the two patients. BMIs are 35 kg/m2 and 41 kg/m2 at present. The fasting glucose is 6.1 mmol/L and 6.2 mmol/L.
All patients received endoscopic ultrasonoscopy and abdominal CT scan every year to find out local recurrence and metastasis.
DISCUSSION
Obesity and type 2 diabetes are likely to be the two greatest public health problems of the coming decade.4 Evidence suggests that bariatric surgery is associated with a 60–80% diabetes remission rate in severely obese persons and that earlier interventions are more likely to provide remission.5 So more and more obese patients prefer to receive bariatric surgery in order to control their obesity and diabetes.6,7
GISTs of the stomach are one of the most important submucosal tumours, becoming more frequently encountered because of the rising incidence of upper gastrointestinal endoscopy, especially the ultrasound endoscopy. With this kind of technique, GISTs less than 2 cm in diameter could be easily diagnosed. The National Comprehensive Cancer Network and the European Society of Medical Oncology released consensus statements in 2004 questioning the appropriateness of minimally invasive techniques for managing GISTs of the stomach due to concerns of tumour rupture and spillage and ability to obtain an negative microscopic margin resection.8,9 According to their recommendations, our LSG in treatment of GISTs is acceptable. The estimated incidence of GISTs is approximately 10–20 per million people per year.3,10 It is difficult to predict the malignancy potential of GISTs solely based on clinical and pathological findings other than the presence of obvious metastasis at surgery.11,12 Many of the previous studies considered that a single factor or even a combination of two factors was not sufficient to reliably predict the exact behaviour of GISTs.13,14 Currently, no accepted staging system exists for GIST. Most of the pathologists consider multiple parameters to predict the biological behaviour of the GIST. Cellular pleomorphism and ploidy are not considered as predictors of malignancy in GISTs.15 Size and mitotic count are the most useful predictors of the malignant behaviour.16 Tumours <5 cm are usually benign while those >5 cm are malignant.12,15,16
Endoscopic ultrasonoscopy and CT scan are important in the detection of recurrence and metastasis. In our patients, no local recurrence and metastasis was found in the first year.17 The early diagnosis of GIST is very important. Endoscopic ultrasonoscopy can be used to find GISTs <1 cm.
LSG is thought to be the best choice for obese patients combined with GISTs. We are not sure if bypass and lap-band surgery will stimulate the GISTs. LSG can remove tumours and excess stomach away at the same time. As a result, the patients all recovered successfully.
LEARNING POINTS
Laparoscopic sleeve gastrectomy (LSG) is an effective and safe method in treating gastrointestinal stromal tumours (GISTs) in morbid obese patients.
LSG is an effective method in controlling body weights and fasting glucose of morbid obese patients.
Endoscopic ultrasonography can help to find GISTs at the early stage.
Footnotes
Competing interests: none.
Patient consent: Patient/guardian consent was obtained for publication.
REFERENCES
- 1.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001; 414: 782–7 [DOI] [PubMed] [Google Scholar]
- 2.Lau S, Tam KF, Kam CK, et al. Imaging of gastrointestinal stromal tumour (GIST). Clin Radiology 2004; 59: 487–98 [DOI] [PubMed] [Google Scholar]
- 3.Gold JS, Dematteo RP. Combined surgical and molecular therapy: the gastrointestinal stromal tumor model. Ann Surg 2006; 244: 176–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study Group Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. UK Prospective Diabetes Study Group. BMJ 1998; 317: 713–20 [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon JB, Pories WJ, O’Brien PE, et al. Surgery as an effective early intervention for diabesity: why the reluctance? Diabetes Care 2005; 28: 472–4 [DOI] [PubMed] [Google Scholar]
- 6.Steinbrook R. Surgery for severe obesity. New Engl J Med 2004; 350: 1075–9 [DOI] [PubMed] [Google Scholar]
- 7.Pinkney JH, Sjostrom CD, Gale EA. Should surgeons treat diabetes in severely obese people? Lancet 2001; 357: 1357–9 [DOI] [PubMed] [Google Scholar]
- 8.Blay JY, Bonvalot S, Casali P, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20–21 March 2004, under the auspices of ESMO. Ann Oncol 2005; 16: 566–78 [DOI] [PubMed] [Google Scholar]
- 9.Demetri GD, Benjamin RS, Blanke CD, et al. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw 2007; 5: S1–9; quiz S30 [PubMed] [Google Scholar]
- 10.Levy AD, Remotti HE, Thompson WM, et al. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics 2003; 23: 283–304, 456; quiz 532 [DOI] [PubMed] [Google Scholar]
- 11.Novitsky YW, Kercher KW, Sing RF, et al. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg 2006; 243: 738–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dougherty MJ, Compton C, Talbert M, et al. Sarcomas of the gastrointestinal tract. Separation into favorable and unfavorable prognostic groups by mitotic count. Ann Sur 1991; 214: 569–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bucher P, Villiger P, Egger JF, et al. Management of gastrointestinal stromal tumors: from diagnosis to treatment. Swiss Med Wkly 2004; 134: 145–53 [DOI] [PubMed] [Google Scholar]
- 14.Chan KH, Chan CW, Chow WH, et al. Gastrointestinal stromal tumors in a cohort of Chinese patients in Hong Kong. World J Gastroenterol 2006; 12: 2223–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pidhorecky I, Cheney RT, Kraybill WG, et al. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol 2000; 7: 705–12 [DOI] [PubMed] [Google Scholar]
- 16.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol 2002; 10: 81–9 [DOI] [PubMed] [Google Scholar]
- 17.Schubert D, Kuhn R, Nestler G, et al. Laparoscopic-endoscopic rendezvous resection of upper gastrointestinal tumors. Dig Dis 2005; 23: 106–12 [DOI] [PubMed] [Google Scholar]