Abstract
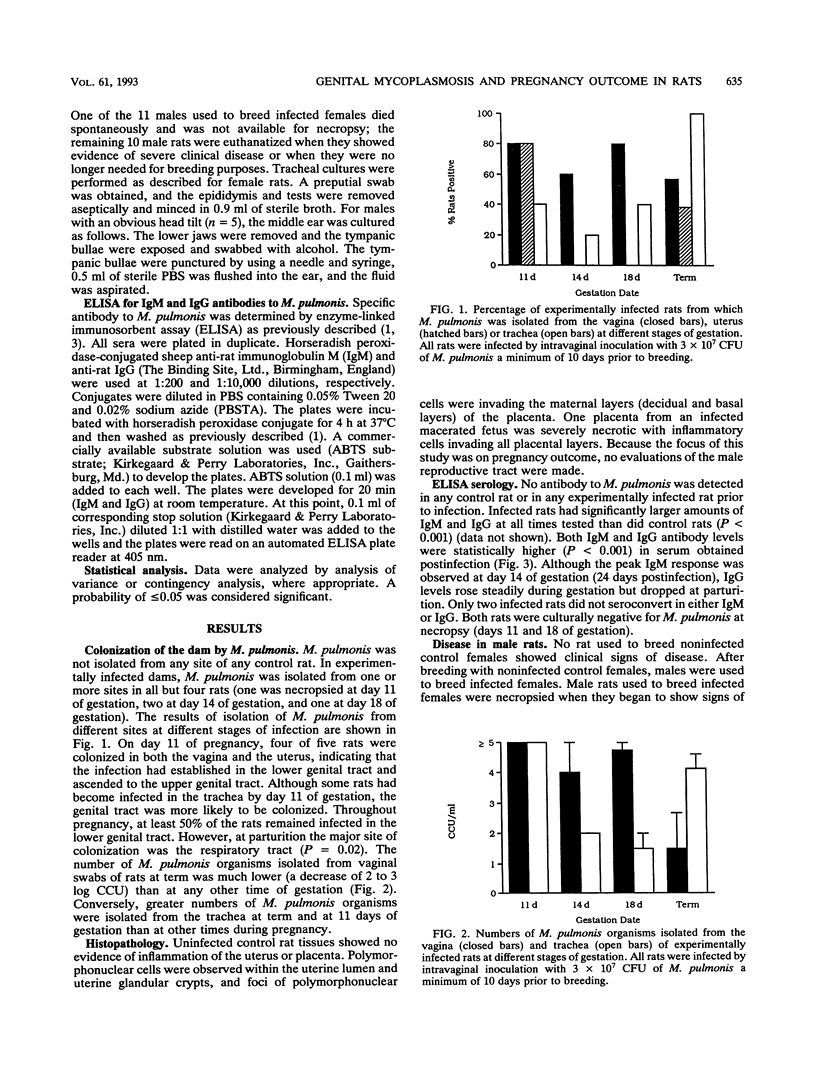
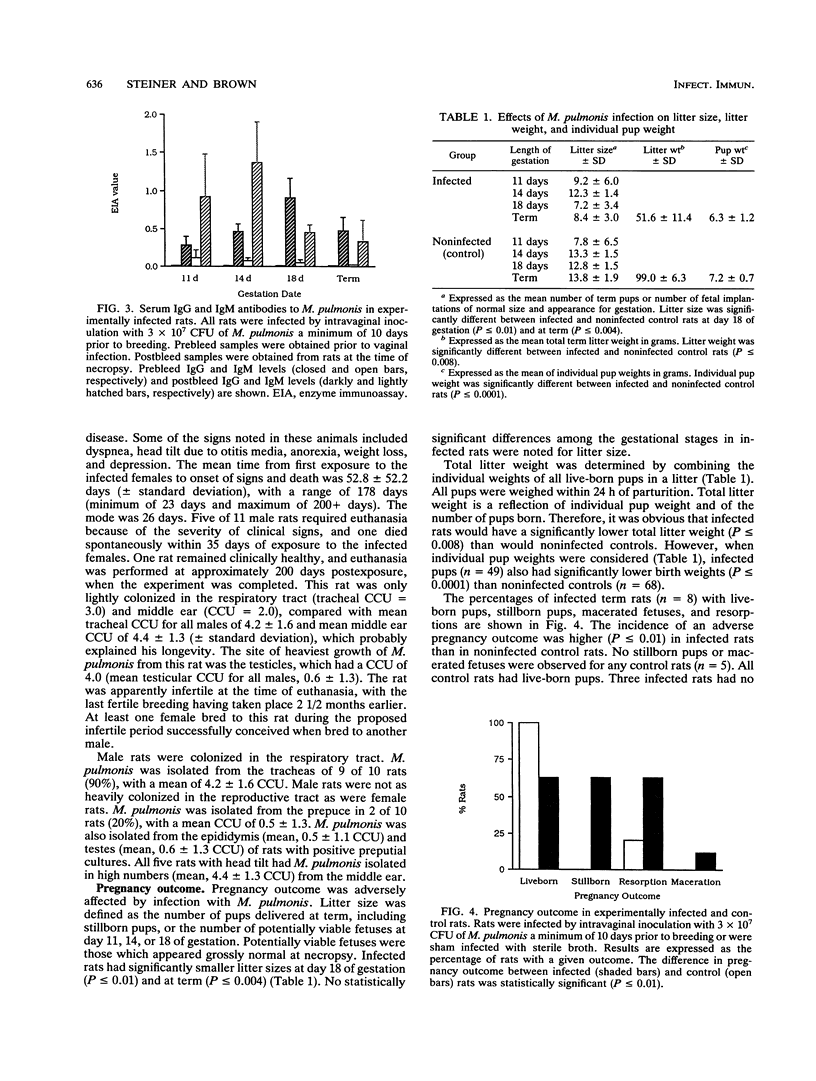
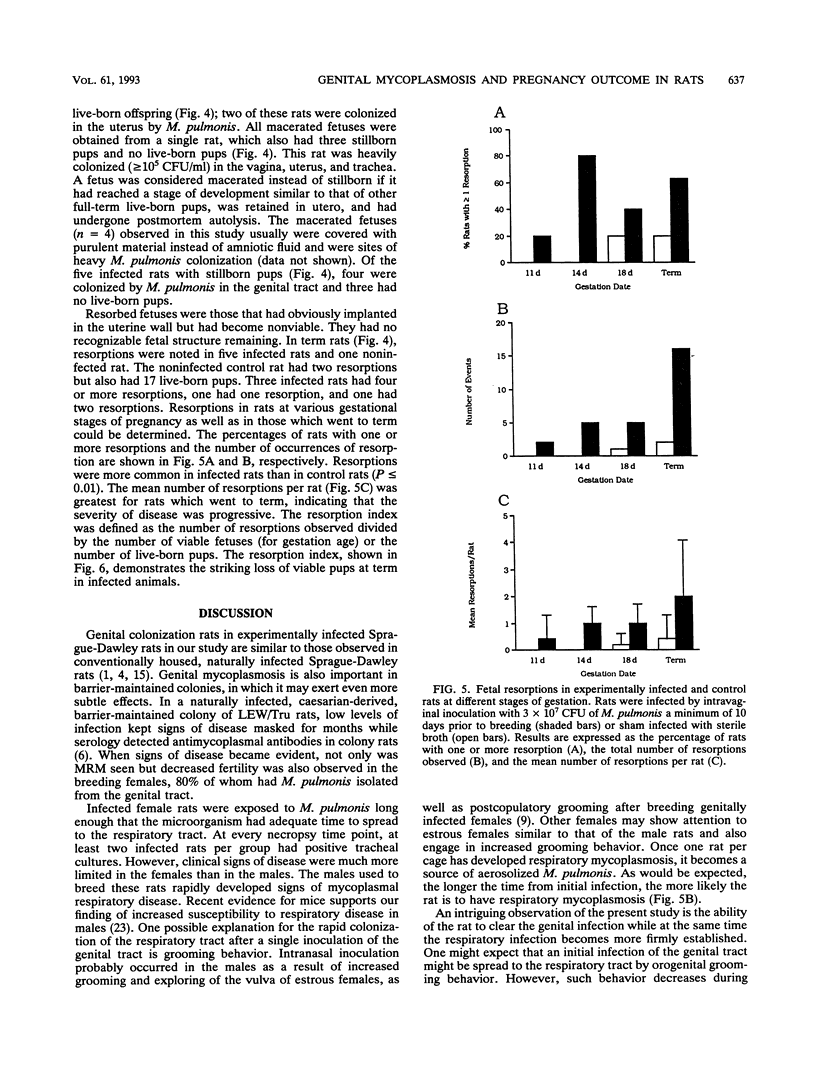
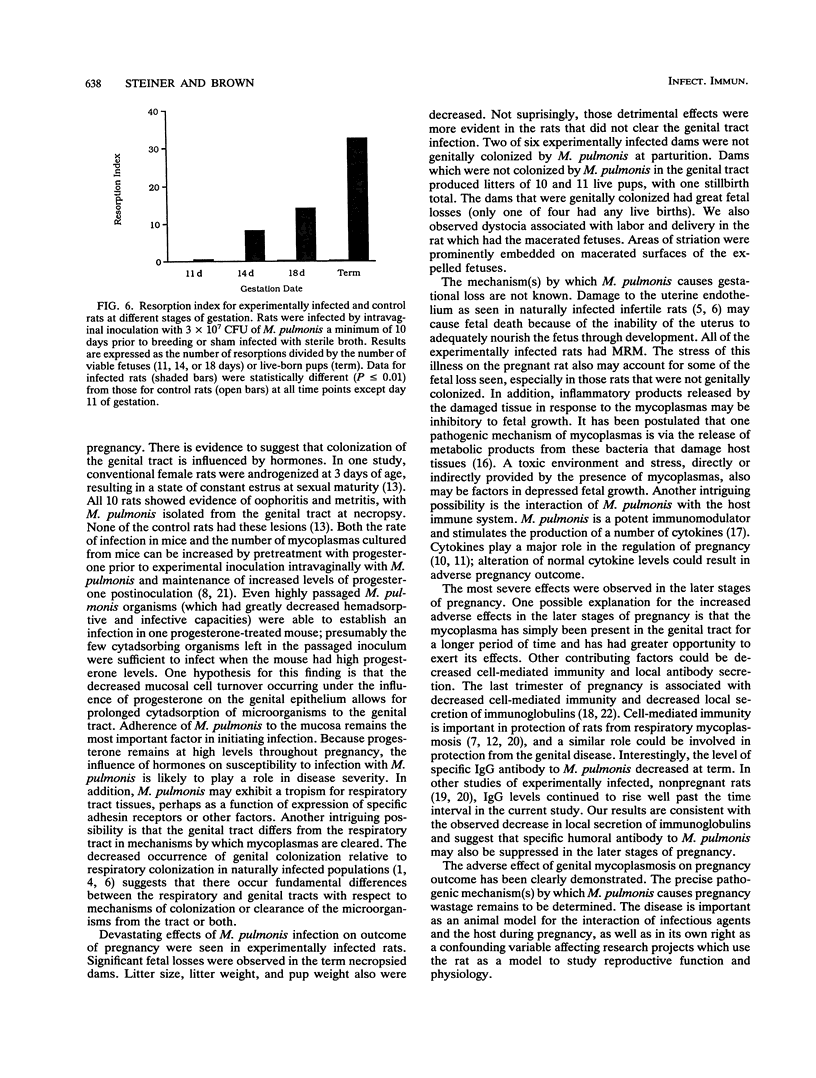
Specific-pathogen-free (SPF) female Sprague-Dawley rats were infected by intravaginal inoculation with 3 x 10(7) CFU of Mycoplasma pulmonis X1048 in 0.1 ml of Frey's broth or with an equal volume of sterile Frey's broth. A minimum of 10 days postinfection, rats were bred to noninfected males. Rats were necropsied at days 11, 14, and 18 of gestation and within 24 h of parturition. Throughout pregnancy, at least 50% of rats remained infected in the lower genital tract. At parturition, the major site of colonization was the respiratory tract (P = 0.02). M. pulmonis was not isolated from any site of any control rat. Pregnancy outcome was adversely affected by infection with M. pulmonis. Infected rats had significantly smaller litter sizes at day 18 of gestation (P < or = 0.01) and at term (P < or = 0.004). No statistically significant differences among the gestational stages in infected rats were noted for litter size. Total litter weight is a reflection of individual pup weight and of the number of pups born. Therefore, it was obvious that infected rats would have a significantly lower (P < or = 0.008) total litter weight than noninfected controls. However, when individual pup weights were considered, infected pups (n = 49) also had significantly lower (P < or = 0.0001) birth weights than did noninfected controls (n = 68). The incidence of an adverse pregnancy outcome at term (stillbirths, macerated fetuses, or resorptions) was higher (P < or = 0.01) in infected rats than in noninfected control rats. No stillborn pups or macerated fetuses were observed in any control term rats (n = 5). All control rats had live-born pups. Three infected rats had no live-born offspring. Resorptions were more common in infected rats than in control rats (P < or = 0.01). The mean number of resorptions per rat was greater in rats which went to term than in rats necropsied during gestation, indicating that the severity of disease was progressive. The rat is frequently the laboratory animal of choice for a wide variety of reproductive studies, and the experimental parameters that are most often measured (litter size, pup weight, and neonatal survival) were all adversely affected by genital mycoplasmosis. Genital mycoplasmosis is important as an animal model for the interaction of infectious agents and the host during pregnancy as well as in its own right as a confounding variable affecting research projects which use the rat as a model to study reproductive function and physiology.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. B., Reyes L. Immunoglobulin class- and subclass-specific responses to Mycoplasma pulmonis in sera and secretions of naturally infected Sprague-Dawley female rats. Infect Immun. 1991 Jun;59(6):2181–2185. doi: 10.1128/iai.59.6.2181-2185.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell G. H., Lindsey J. R., Davis J. K. Respiratory and genital mycoplasmosis of laboratory rodents: implications for biomedical research. Isr J Med Sci. 1981 Jul;17(7):548–554. [PubMed] [Google Scholar]
- Cox N. R., Davidson M. K., Davis J. K., Lindsey J. R., Cassell G. H. Natural mycoplasmal infections in isolator-maintained LEW/Tru rats. Lab Anim Sci. 1988 Aug;38(4):381–388. [PubMed] [Google Scholar]
- Davis J. K., Simecka J. W., Williamson J. S., Ross S. E., Juliana M. M., Thorp R. B., Cassell G. H. Nonspecific lymphocyte responses in F344 and LEW rats: susceptibility to murine respiratory mycoplasmosis and examination of cellular basis for strain differences. Infect Immun. 1985 Jul;49(1):152–158. doi: 10.1128/iai.49.1.152-158.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr P. M., Taylor-Robinson D. Enhancement of experimental Mycoplasma pulmonis infection of the mouse genital tract by progesterone treatment. J Hyg (Lond) 1984 Apr;92(2):139–144. doi: 10.1017/s0022172400064147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B. L., Korinek E., Brennan P. Postcopulatory genital grooming in male rats: prevention of sexually transmitted infections. Physiol Behav. 1987;41(4):321–325. doi: 10.1016/0031-9384(87)90395-7. [DOI] [PubMed] [Google Scholar]
- Hunt J. S. Cytokine networks in the uteroplacental unit: macrophages as pivotal regulatory cells. J Reprod Immunol. 1989 Sep;16(1):1–17. doi: 10.1016/0165-0378(89)90002-8. [DOI] [PubMed] [Google Scholar]
- Hunt J. S. Macrophages in human uteroplacental tissues: a review. Am J Reprod Immunol. 1989 Nov-Dec;21(3-4):119–122. doi: 10.1111/j.1600-0897.1989.tb01015.x. [DOI] [PubMed] [Google Scholar]
- Lai W. C., Bennett M., Pakes S. P., Kumar V., Steutermann D., Owusu I., Mikhael A. Resistance to Mycoplasma pulmonis mediated by activated natural killer cells. J Infect Dis. 1990 Jun;161(6):1269–1275. doi: 10.1093/infdis/161.6.1269. [DOI] [PubMed] [Google Scholar]
- Leader R. W., Leader I., Witschi E. Genital mycoplasmosis in rate treated with testosterone propionate to produce constant estrus. J Am Vet Med Assoc. 1970 Dec 1;157(11):1923–1925. [PubMed] [Google Scholar]
- Lindsey J. R., Davidson M. K., Schoeb T. R., Cassell G. H. Mycoplasma pulmonis-host relationships in a breeding colony of Sprague-Dawley rats with enzootic murine respiratory mycoplasmosis. Lab Anim Sci. 1985 Dec;35(6):597–608. [PubMed] [Google Scholar]
- Razin S. Mycoplasmas: the smallest pathogenic procaryotes. Isr J Med Sci. 1981 Jul;17(7):510–515. [PubMed] [Google Scholar]
- Ruuth E., Praz F. Interactions between mycoplasmas and the immune system. Immunol Rev. 1989 Dec;112:133–160. doi: 10.1111/j.1600-065x.1989.tb00556.x. [DOI] [PubMed] [Google Scholar]
- Schuurs A. H., Verheul H. A. Effects of gender and sex steroids on the immune response. J Steroid Biochem. 1990 Feb;35(2):157–172. doi: 10.1016/0022-4731(90)90270-3. [DOI] [PubMed] [Google Scholar]
- Simecka J. W., Cassell G. H. Serum antibody and cellular responses in LEW and F344 rats after immunization with Mycoplasma pulmonis antigens. Infect Immun. 1987 Mar;55(3):731–735. doi: 10.1128/iai.55.3.731-735.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simecka J. W., Davis J. K., Cassell G. H. Serum antibody does not account for differences in the severity of chronic respiratory disease caused by Mycoplasma pulmonis in LEW and F344 rats. Infect Immun. 1989 Nov;57(11):3570–3575. doi: 10.1128/iai.57.11.3570-3575.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Furr P. M. The interplay of host and organism factors in infection of the mouse genital tract by Mycoplasma pulmonis. J Hyg (Lond) 1985 Aug;95(1):7–14. doi: 10.1017/s0022172400062227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Pregnancy-associated depression of cell-mediated immunity. Rev Infect Dis. 1984 Nov-Dec;6(6):814–831. doi: 10.1093/clinids/6.6.814. [DOI] [PubMed] [Google Scholar]


