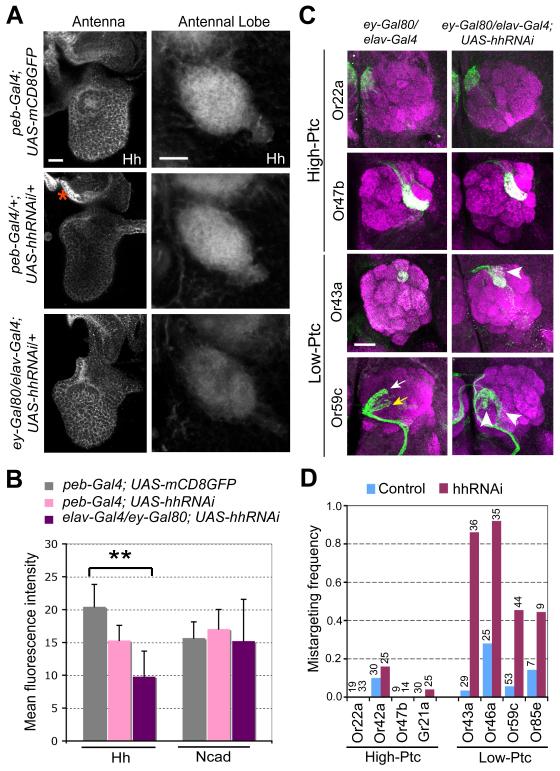
Figure 4. Brain-derived Hh Is Required for Axon Targeting of Low-Ptc ORN Classes.
(A-B) Hh protein levels in antennae and antennal lobes after RNAi-mediated Hh knockdown in the periphery or central brain. (A) 36h APF pupal antennae (left) and antennal lobes (right) were stained for Hh protein in genotypes of flies labeled on the left. A region lacking peb-Gal4 expression is marked by an asterisk and serves as control for the knockdown effect of hhRNAi. (B) Quantification of relative Hh expression in the antennal lobes. N-cadherin expression in the same antennal lobes was used as control. n=5-6 for each condition. Data are represented as mean±SD. **, P<0.005 (unpaired T-test). All others: not significantly different from control (peb-Gal4;UAS-mCD8GFP).
(C-D) Brain-derived Hh is required for axon targeting of low-Ptc but not high-Ptc ORN classes. (C) Axons of two high-Ptc classes do not exhibit targeting defects (top two rows). Axons of two low-Ptc ORN classes exhibit targeting defects (arrowheads in bottom two rows) when Hh in brain neurons but not ORNs was knocked down during ORN axon targeting. Arrows, normal target glomerulus for Or59c (yellow) and a secondary glomerular target (white). (D) Quantification of axon targeting phenotypes of 8 ORN classes from flies with brain-derived Hh knocked down. Numbers of brains analyzed are shown on top.
Figure S4 provides more data on Hh expression in the pupal brain. Figure S5 provides evidence that Hh signaling is not required for projection neuron dendrite development. Figure S6A provides a comparison of glomerular mistargeting preference of reducing Hh from the brain (Figure 4) and removing Smo or Ihog (Table 1) from ORNs.