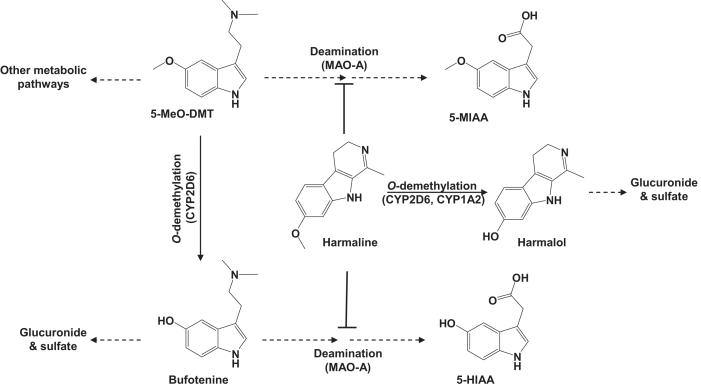
Figure 1.
5-MeO-DMT biotransformation and the metabolic interactions with harmaline. O-demethylation of 5-MeO-DMT by CYP2D6 produces an active metabolite bufotenine. Both 5-MeO-DMT and bufotenine are readily deaminated by MAO-A to indoleacetic acid derivatives. The MAOI harmaline, which is inactivated by CYP2D6, blocks the deamination metabolism of 5-MeO-DMT and bufotenine.