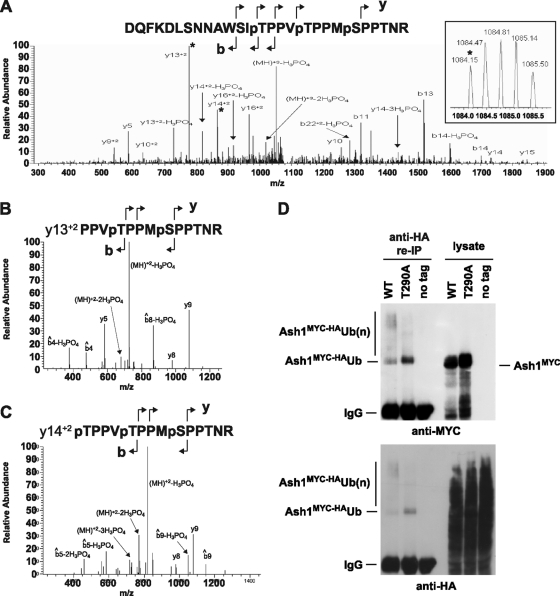
FIG. 4.
In vivo phosphorylation and ubiquitination of Ash1. (A) MS/MS spectrum from the triply phosphorylated peptide D273-R299 (DQFKDLSNNAWSITPPVTPPMSPPTNR). The inset shows the precursor mass from the MS spectrum. The transition ions Y132+ and Y142+, which correspond to the peaks fragmented in panels B and C, are marked with asterisks. (B) MS/MS/MS from the Y132+ fragment (PPVTPPMSPPTNR) shows neutral losses of only 2 phosphate groups, with similar fragmentation patterns observed for both MS/MS/MS spectra. (C) MS/MS/MS from the Y142+ fragment (TPPVTPPMSPPTNR) shows neutral loss of all 3 phosphate groups. (D) Immunopurified anti-MYC complexes from strains bearing an integrated HA-tagged allele of ubiquitin alone or in combination with either wild-type (WT) ash1::GAL1-ASH1MYC or ash1::GAL1-ASH1MYCT290A were denatured by boiling in SDS-PAGE sample buffer, diluted in nondenaturing buffer, recaptured on anti-HA resin, and then probed with either anti-MYC or anti-HA antibody as indicated. Input lysates were probed in parallel.