FIG. 7.
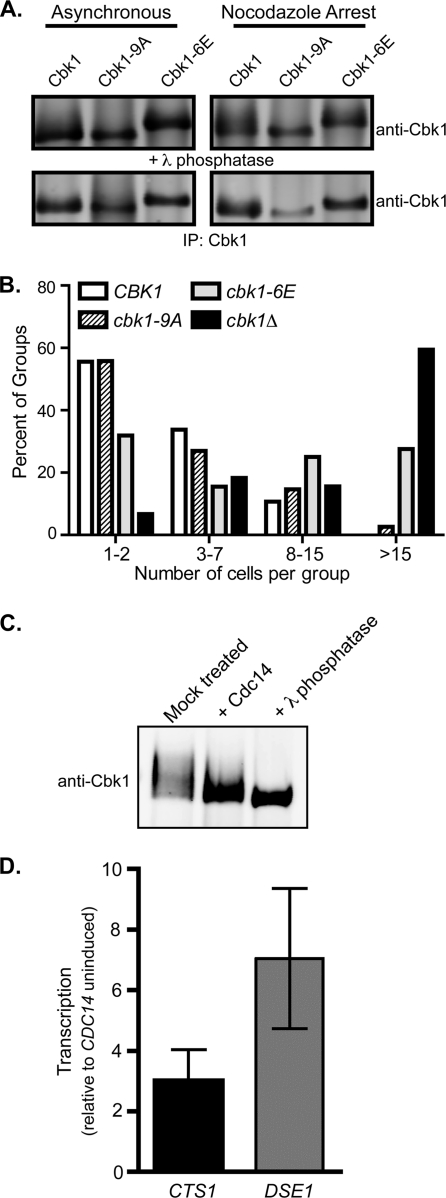
Removal of CDK phosphorylation on Cbk1 by Cdc14 may activate Cbk1 independently of the MEN. (A) Mutation of potential CDK phosphorylation sites on Cbk1. Nine potential CDK1 phosphorylation sites on Cbk1 were mutated to alanine (cbk1-9A), or six of these sites in the C terminus of Cbk1 were mutated to glutamic acid (cbk1-6E). Cbk1 was immunoprecipitated from asynchronous cells (left) or nocodazole-arrested cells (right). Both alleles were expressed at levels similar to the wild-type protein. The Cbk1-9A protein exhibited less electrophoretic mobility shifting compared to the wild-type protein. Lambda phosphatase-treated protein is shown below. (B) Glutamic acid substitution at CDK sites in Cbk1 is inhibitory. The number of connected cells in a group was determined for the indicated strains. The cbk1-9A allele promoted wild-type separation, while the cbk1-6E allele had a defect in cell separation. (C) Cdc14 can dephosphorylate Cbk1 in vitro. Immunoprecipitated Cbk1 was treated in vitro with bacterially purified Cdc14 or treated with lambda phosphatase as a control for complete dephosphorylation. (D) Overexpression of Cdc14 can activate Cbk1 and Ace2 independently of MEN. cdc15-2 cells containing a plasmid for PrGAL-GST-CDC14 were arrested at 37°C. Cells were maintained at the arrest, and CDC14 was induced (galactose) or uninduced (glucose). Cells were harvested, and QPCR was performed to determine transcript levels of CTS1 and DSE1 relative to uninduced cells. Standard errors of the means from three independent trials are shown.