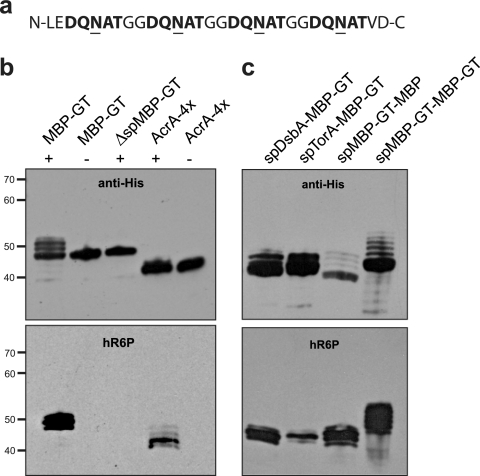
FIG. 1.
Glycosylation tag for making recombinant glycoproteins in E. coli. (a) The GT is comprised of four consecutive D-X1-N-X2-T sequons that are efficiently glycosylated in bacteria. (b) Western blot analysis of (from left to right) MBP with C-terminal GT (MBP-GT), mature MBP lacking its native signal peptide with C-terminal GT (ΔspMBP-GT), and the native C. jejuni glycoprotein AcrA engineered with two additional glycan acceptor sites (AcrA-4×). Proteins were expressed in cells carrying pACYCpgl (+) or pACYCpglmut (−). (c) Western blot analysis of (from left to right) MBP-GT with its native signal peptide replaced with the signal peptide of DsbA or TorA, MBP harboring an N-terminal GT following its native signal sequence (spMBP-GT-MBP), and MBP carrying both N- and C-terminal GT sequences (spMBP-GT-MBP-GT). Each was expressed in cells carrying pACYCpgl. Proteins were purified from cell lysates by Ni-NTA affinity chromatography. The same amount of protein was loaded in each lane. Blots were probed with anti-His (top) or hR6P (bottom) antibodies.