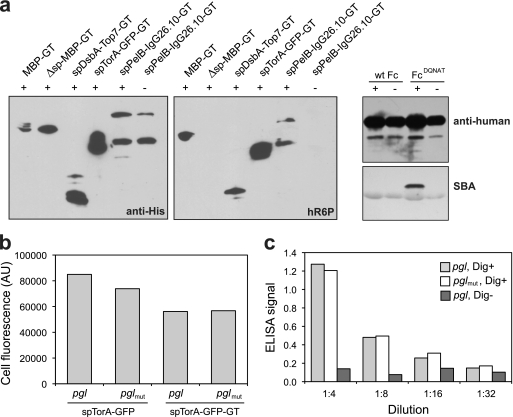
FIG. 3.
Glycosylation of diverse recombinant proteins in glycoengineered E. coli. (a) Western blot analysis of (from left to right) MBP-GT with or without its native signal peptide, TOP7-GT with the DsbA signal peptide, GFP-GT with the TorA signal peptide, and the murine anti-digoxin IgG 26.10 with a PelB signal peptide on the light chain and a DsbA signal peptide on the heavy chain, which was also appended with the GT. Proteins were expressed in cells carrying pACYCpgl (+) or pACYCpglmut (−). Blots were probed with anti-His (left) or hR6P (right) antibodies. Western blot analysis of wild-type Fc (wt Fc) and FcDQNAT was conducted with anti-human antibodies (top) and SBA (bottom). (b) Fluorescence of cells expressing spTorA-GFP or spTorA-GFP-GT in pgl or pglmut cells as indicated. Data are the averages of results from three replicate experiments, and the standard error was less than 5%. (c) ELISA signals for plates coated with BSA-digoxin conjugate (Dig+) and probed with 26.10 IgG purified from pgl or pglmut cells. Control wells without BSA-digoxin (Dig−) were incubated with 26.10 IgG purified from pgl cells.