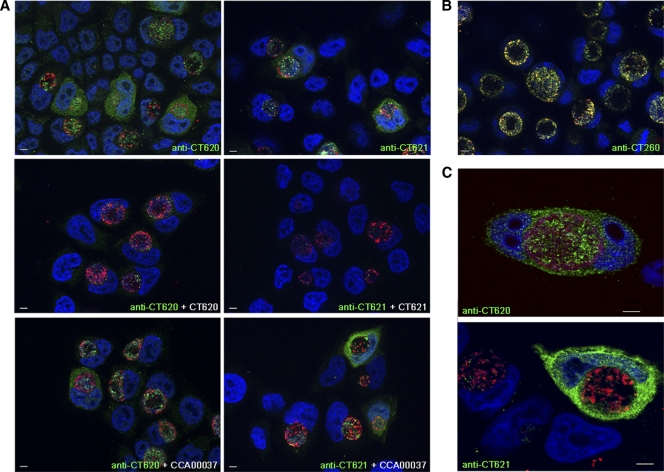
FIG. 4.
The C. trachomatis DUF582 proteins CT620 and CT621 are detected in the host cytoplasm during chlamydial infection. HeLa cells were infected with C. trachomatis L2 and fixed 31 h later. (A) C. trachomatis DUF582 proteins were immunolabeled with anti-CT620 and anti-CT621 as indicated, and bacteria were detected with a mouse anti-Hsp60 antibody. Coverslips were subsequently incubated with Alexa 488-conjugated anti-rabbit antibody to detect the DUF582 proteins (green) and with Cy5-conjugated anti-mouse antibody for Hsp60 (red). Host cell nuclei and bacterial DNA were stained with Hoechst stain (blue). For antibody competition, anti-CT620 and anti-CT621 were incubated in the presence of an excess of His-CT620, His-CT621, or CCA00037-His (an irrelevant His-tagged protein), as indicated. Arrows point to cells in which cytoplasmic staining for CT620 and CT621 is observed. Note that in addition to the cytoplasmic staining, CT620 and CT621 also localize to the lumen of the chlamydial inclusion with only little colocalization with the bacterial protein Hsp60. (B) Infected cells were labeled with antibodies to the chlamydial chaperones CT260 (green) and Hsp60 (red), which colocalize. (C) C. trachomatis DUF582 proteins were immunolabeled with anti-CT620 and anti-CT621 as indicated, and bacteria were detected with a mouse anti-MOMP antibody. Coverslips were subsequently incubated with Alexa 488-conjugated anti-rabbit antibody to detect the DUF582 proteins (green) and with Cy5-conjugated anti-mouse antibody for MOMP (red). Host cell nuclei and bacterial DNA were stained with Hoechst stain (blue). Images were acquired using an ApoTome fluorescence microscope. Bars, 5 μm.