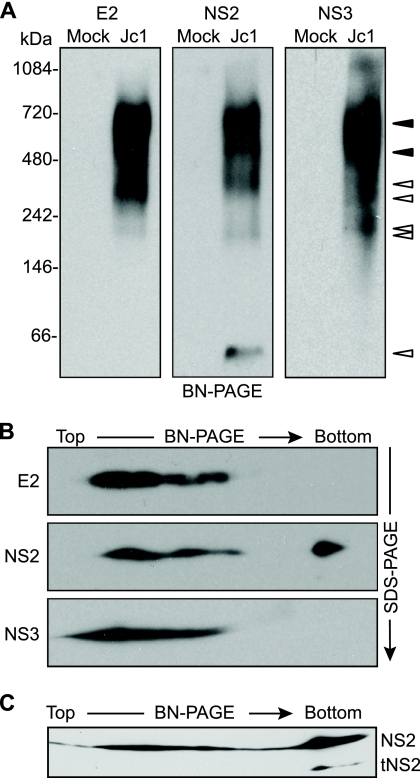
FIG. 3.
E2, NS2, and NS3 form discrete, membrane-associated high-molecular-mass complexes. (A) BN-PAGE separation of E2-, NS2-, and NS3-containing protein complexes. Microsomes were prepared at 48 h postelectroporation of Huh-7.5 cells with Jc1 RNA or cells transfected without RNA (Mock). Proteins were solubilized in 0.5% (vol/vol) digitonin, separated under native conditions on a 4 to 16% Bis-Tris polyacrylamide gel, and transferred to PVDF. The blot was cut into three strips and immunoblotted for E2, NS2, and NS3. Open arrowheads indicate bands that were specific for a given protein; closed arrowheads indicate bands in which E2, NS2, and NS3 comigrated (see Results). (B) 2D BN/SDS-PAGE analysis of E2-, NS2-, and NS3-containing protein complexes. Microsome-associated protein (20 μg) was solubilized in digitonin and separated in the first dimension by BN-PAGE and in the second dimension on a 10% SDS-PAGE gel. E2, NS2, and NS3 were detected by immunoblotting. (C) Microsome-associated protein (40 μg) was solubilized in digitonin and separated in the first dimension by BN-PAGE and in the second dimension on a 12% SDS-PAGE gel. NS2 was detected by immunoblotting.