FIG. 2.
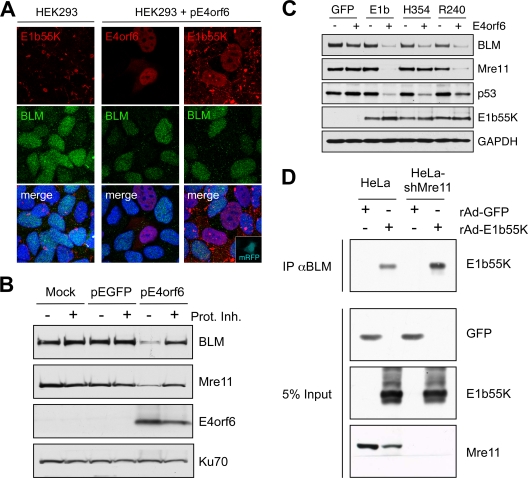
E1b55K and E4orf6 are sufficient for degradation of BLM. (A) Immunofluorescence reveals that in HEK293 cells the BLM protein is located throughout the nucleoplasm and E1b55K is in cytoplasmic aggregates (left). When HEK293 cells were transfected with E4orf6 (15), BLM levels were reduced (middle). In cells transfected with plasmids expressing E4orf6 and monomeric red fluorescent protein (mRFP) (at a 9:1 ratio), transfected cells demonstrated nuclear E1b55K, with reduced BLM levels (right). Cells were fixed for staining (14) at 24 h after transfection, and DAPI (4′,6-diamidino-2-phenylindole) staining indicates the location of the nuclei in all merged images. (B) Expression of E4orf6 by transfection of HEK293 cells demonstrated a proteasome-dependent decrease in BLM levels. Cells were harvested at 20 h posttransfection for analysis by immunoblotting with specific antibodies. Degradation was abrogated by proteasome inhibitors (10 μM MG132 and 1 μM epoxomicin). Mre11 served as a control for degradation, and Ku70 served as a loading control. (C) E1b55K and E4orf6 are sufficient for BLM degradation. E4orf6 was expressed by rAd vector transduction (48) of U2OS cells that stably express GFP or E1b55K (14), and protein levels were assessed by immunoblotting with the indicated antibodies. Antibodies to Mre11 (Genetex) and p53 (Calbiochem) served as controls for degradation, and Ku70 served as a loading control. (D) E1b55K coimmunoprecipitates with BLM in the absence of Mre11. HeLa cells or HeLa-shMre11 cells were infected (MOI of 50) with rAd-GFP and rAd-E1b55K (48) for 24 h, and lysates were subjected to immunoprecipitation with the BLM antibody (IP αBLM). Immunoblotting of the precipitated proteins demonstrated that E1b55K could be pulled down by BLM in lysates from both cells.