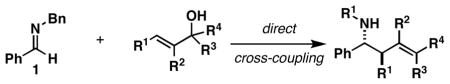
Table 1.
 | |||||
|---|---|---|---|---|---|
| entry | allylic alcohol | yield (%)[a] | (Z:E) | dr | product |
| 1 |
2 |
70 | – | – |
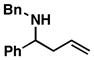 3 |
| 2 |
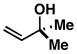 4 |
83 | – | – |
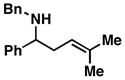 5 |
| 3 |
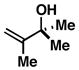 6 |
80 | – | – |
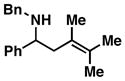 7 |
| 4 |
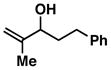 8 |
87 | ≥ 20:1 | – |
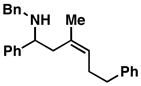 9 |
| 5 |
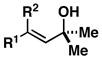 10; R1 = Me; R2 = H |
81 | – | ≥ 20:1 |
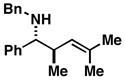 11 |
| 6 | 12; R1 = H; R2 = Me 11 | 68 | – | ≥ 20:1 | |
| 7 |
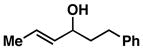 13 |
92 | 1.6:1 | ≥ 20:1[b] |
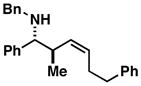 14 |
| 8 |
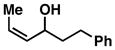 15 |
57 | ≤ 1:20 | ≥ 20:1 |
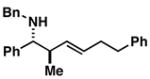 16 |
| 9 |
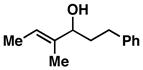 17 |
54 | ≥ 20:1 | ≥ 20:1 |
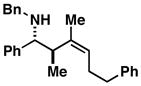 18 |
Reaction conditions: imine (1 eq), Ti(Oi-Pr)4 (1.5 eq), c-C5H9MgCl (3 eq) (−78 to −40 °C), then add lithium alkoxide of allylic alcohol (1.5 eq) in THF (−40 to 0 °C; or −40 °C to rt - entries 6 and 8), then quench with H2O.
Each alkene isomer (Z and E) was identified as the anti-diastereomer (dr ≥ 20:1).
